Unraveling the exceptional kinetics of Zn||organic batteries in hydrated deep eutectic solution
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
Abstract
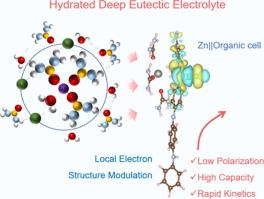
Intuitively, the solvation structure featuring stronger interacted sheath in deep eutectic solution (DES) electrolyte would result in sluggish interfacial charge transfer and intense polarization, which obstructs its practical application in emerging Zn based batteries. Unexpectedly, here we discover a Zn||organic battery with exceptional kinetics properties enabled by a hydrated DES electrolyte, which can render higher discharge capacity, smaller voltage polarization, and faster kinetics of charge transfer in comparison with conventional aqueous 3 M ZnCl2 electrolyte, though its viscosity is two orders of magnitude higher than the latter. The improved kinetics of charge transfer and ion diffusion is demonstrated to originate from the local electron structure regulation of cathode in hydrated DES electrolyte. Furthermore, the DES electrolyte has also been shown to restrict parasitic reaction associated with active water by preferential urea-molecular adsorption on Zn surface and stronger water trapping in solvation structure, giving rise to long-term stable dendrite-free Zn plating/stripping. This work provides a new rationale for understanding electrochemical behaviors of organic cathodes in DES electrolyte, which is conducive to the development of high-performance Zn||organic batteries.
揭示锌||有机电池在水合深共晶溶液中的特殊动力学特性
直观地说,深共晶溶液(DES)电解质中具有较强相互作用鞘的溶解结构会导致界面电荷转移迟缓和极化严重,从而阻碍其在新兴锌基电池中的实际应用。与传统的 3 M ZnCl2 水溶液电解液相比,水合 DES 电解液具有更高的放电容量、更小的电压极化和更快的电荷转移动力学,尽管其粘度比后者高出两个数量级。电荷转移和离子扩散动力学的改善源于水合 DES 电解质中阴极局部电子结构的调节。此外,DES 电解质还通过在 Zn 表面优先吸附尿素分子和加强溶解结构中的水捕获作用,限制了与活性水相关的寄生反应,从而实现了长期稳定的无树枝状 Zn 镀层/剥离。这项工作为理解有机阴极在 DES 电解液中的电化学行为提供了新的理论依据,有利于开发高性能 Zn||有机电池。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


