Integrating Cu+/Cu0 sites on porous nitrogen-doped carbon nanofibers for stable and efficient CO2 electroreduction to multicarbon products
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
Abstract
The Cu+/Cu0 sites of copper-based catalysts are crucial for enhancing the production of multicarbon (C2+) products from electrochemical CO2 reduction reaction (eCO2RR). However, the unstable Cu+ and insufficient Cu+/Cu0 active sites lead to their limited selectivity and stability for C2+ production. Herein, we embedded copper oxide (CuOx) particles into porous nitrogen-doped carbon nanofibers (CuOx@PCNF) by pyrolysis of the electrospun fiber film containing ZIF-8 and Cu2O particles. The porous nitrogen-doped carbon nanofibers protected and dispersed Cu+ species, and its microporous structure enhanced the interaction between CuOx and reactants during eCO2RR. The obtained CuOx@PCNF created more effective and stable Cu+/Cu0 active sites. It showed a high Faradaic efficiency of 62.5% for C2+ products in H-cell, which was 2 times higher than that of bare CuOx (∼31.1%). Furthermore, it achieved a maximum Faradaic efficiency of 80.7% for C2+ products in flow cell. In situ characterization and density functional theory (DFT) calculation confirmed that the N-doped carbon layer protected Cu+ from electrochemical reduction and lowered the energy barrier for the dimerization of *CO. Stable and exposed Cu+/Cu0 active sites enhanced the enrichment of *CO and promoted the C–C coupling reaction on the catalyst surface, which facilitated the formation of C2+ products.
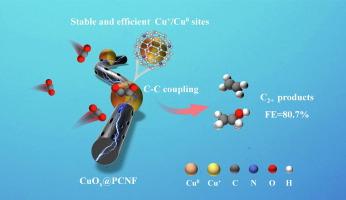
在多孔掺氮碳纳米纤维上整合 Cu+/Cu0 位点,稳定高效地将二氧化碳电还原为多碳产品
铜基催化剂的 Cu+/Cu0 位点对于提高电化学二氧化碳还原反应(eCO2RR)中多碳(C2+)产物的产量至关重要。然而,不稳定的 Cu+ 和不足的 Cu+/Cu0 活性位点导致它们在生产 C2+ 时的选择性和稳定性有限。在此,我们通过热解含有 ZIF-8 和 Cu2O 颗粒的电纺纤维膜,将氧化铜(CuOx)颗粒嵌入多孔掺氮碳纳米纤维(CuOx@PCNF)中。多孔掺氮碳纳米纤维保护并分散了 Cu+ 物种,其微孔结构增强了 eCO2RR 过程中 CuOx 与反应物之间的相互作用。获得的 CuOx@PCNF 创造了更有效、更稳定的 Cu+/Cu0 活性位点。在 H-cell 中,C2+ 产物的法拉第效率高达 62.5%,是裸 CuOx(31.1%)的 2 倍。此外,在流动池中,C2+ 产物的法拉第效率最高可达 80.7%。原位表征和密度泛函理论(DFT)计算证实,掺杂 N 的碳层保护了 Cu+ 免受电化学还原,并降低了 *CO 的二聚化能垒。稳定和暴露的 Cu+/Cu0 活性位点增强了 *CO 的富集,促进了催化剂表面的 C-C 偶联反应,从而促进了 C2+ 产物的形成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


