Sulfur doping and oxygen vacancy in In2O3 nanotube co-regulate intermediates of CO2 electroreduction for efficient HCOOH production and rechargeable Zn-CO2 battery
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
Abstract
By manipulating the distribution of surface electrons, defect engineering enables effective control over the adsorption energy between adsorbates and active sites in the CO2 reduction reaction (CO2RR). Herein, we report a hollow indium oxide nanotube containing both oxygen vacancy and sulfur doping (Vo-Sx-In2O3) for improved CO2-to-HCOOH electroreduction and Zn-CO2 battery. The componential synergy significantly reduces the *OCHO formation barrier to expedite protonation process and creates a favorable electronic micro-environment for *HCOOH desorption. As a result, the CO2RR performance of Vo-Sx-In2O3 outperforms Pure-In2O3 and Vo-In2O3, where Vo-S53-In2O3 exhibits a maximal HCOOH Faradaic efficiency of 92.4% at −1.2 V vs. reversible hydrogen electrode (RHE) in H-cell and above 92% over a wide window potential with high current density (119.1 mA cm−2 at −1.1 V vs. RHE) in flow cell. Furthermore, the rechargeable Zn-CO2 battery utilizing Vo-S53-In2O3 as cathode shows a high power density of 2.29 mW cm−2 and a long-term stability during charge–discharge cycles. This work provides a valuable perspective to elucidate co-defective catalysts in regulating the intermediates for efficient CO2RR.
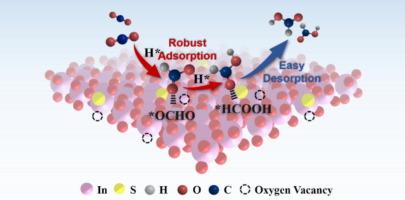
In2O3 纳米管中的硫掺杂和氧空位共同调节 CO2 电还原的中间产物,实现高效 HCOOH 生产和可充电 Zn-CO2 电池
通过操纵表面电子的分布,缺陷工程可以有效控制二氧化碳还原反应(CO2RR)中吸附剂与活性位点之间的吸附能。在此,我们报告了一种含有氧空位和硫掺杂的空心氧化铟纳米管(Vo-Sx-In2O3),用于改进 CO2 到 HCOOH 的电还原和 Zn-CO2 电池。这种成分协同作用大大降低了 *OCHO 的形成障碍,从而加快了质子化过程,并为 *HCOOH 的解吸创造了有利的电子微环境。因此,Vo-Sx-In2O3 的 CO2RR 性能优于 Pure-In2O3 和 Vo-In2O3,其中 Vo-S53-In2O3 在氢电池中与可逆氢电极(RHE)相比,在 -1.2 V 时的 HCOOH 法拉第效率最高可达 92.4%,而在流动电池中,在宽窗口电位下的电流密度较高(与 RHE 相比,在 -1.1 V 时为 119.1 mA cm-2),其 CO2RR 效率高于 92%。此外,利用 Vo-S53-In2O3 作为阴极的可充电 Zn-CO2 电池显示出 2.29 mW cm-2 的高功率密度和充放电循环过程中的长期稳定性。这项工作为阐明共缺陷催化剂在调节高效 CO2RR 的中间产物方面提供了一个有价值的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


