Influence of surface properties of transition metal oxides for permanganate activation: Key role of Lewis acid sites
IF 5.7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Permanganate (PM) activation to remove contaminants in aqueous solution has gained increasing interest, and effective approaches to enhance its oxidation ability are becoming one of the hot spots. Transition metal oxides (TMOs) stand out as the most potential catalysts, but have received little attention in this area until now. Their mechanism towards PM activation also remains controversial. In this study, a series of TMOs with diverse surface properties were synthesized, and their effectiveness was compared. It was observed that α-MnO2, NiO and Co3O4 exhibited significantly higher rates of degrading sulfadiazine, levofloxacin and phenol through PM activation than CeO2, α-Fe2O3 and CuO. A performance evolution depended on the surface Lewis acid (LA) strength was established, while a less consistent correlation was observed between the degradation rate and other surface properties. Based on electrochemical analysis and density functional theory calculations, the spontaneous adsorption of MnO4- anions on LA sites in TMOs with an increase in oxidation potential of PM was further confirmed. This research would deepen the understanding of PM activation mechanism, and offer new guidance in the design of more efficient catalysts.
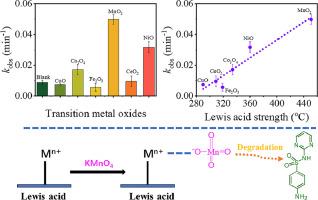
过渡金属氧化物表面性质对高锰酸盐活化的影响:路易斯酸位点的关键作用
利用高锰酸盐(PM)活化去除水溶液中的污染物越来越受到人们的关注,而提高其氧化能力的有效方法正成为研究热点之一。过渡金属氧化物(TMOs)是最具潜力的催化剂,但迄今为止在这一领域却很少受到关注。它们激活 PM 的机制也仍存在争议。本研究合成了一系列具有不同表面性质的过渡金属氧化物,并比较了它们的有效性。结果表明,α-MnO2、NiO 和 Co3O4 通过 PM 活化降解磺胺嘧啶、左氧氟沙星和苯酚的速率明显高于 CeO2、α-Fe2O3 和 CuO。降解率与其他表面特性之间的相关性不那么一致。基于电化学分析和密度泛函理论计算,进一步证实了 MnO4- 阴离子自发吸附在 TMOs 的 LA 位点上,并随着 PM 氧化电位的增加而增加。这项研究将加深对 PM 活化机理的理解,并为设计更高效的催化剂提供新的指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Surfaces and Interfaces
Chemistry-General Chemistry
CiteScore
8.50
自引率
6.50%
发文量
753
审稿时长
35 days
期刊介绍:
The aim of the journal is to provide a respectful outlet for ''sound science'' papers in all research areas on surfaces and interfaces. We define sound science papers as papers that describe new and well-executed research, but that do not necessarily provide brand new insights or are merely a description of research results.
Surfaces and Interfaces publishes research papers in all fields of surface science which may not always find the right home on first submission to our Elsevier sister journals (Applied Surface, Surface and Coatings Technology, Thin Solid Films)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


