Enhanced electrochemical oxidation of benzene and toluene in aqueous environments using Sb–SnO2-doped TiO2 nanotubes modified by hydrophobic polytetrafluoroethylene (PTFE)
IF 4.8
2区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
Progress in Natural Science: Materials International
Pub Date : 2024-10-01
DOI:10.1016/j.pnsc.2024.07.017
引用次数: 0
Abstract
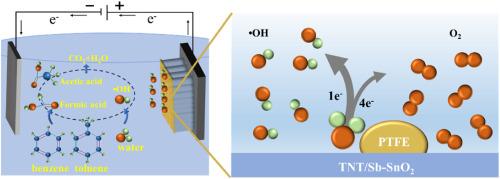
The presence of benzene and toluene in groundwater poses a serious threat to both aquatic ecosystems and human health. The electrochemical removal of these contaminants is primarily hindered by the low generation yield of reactive oxygen species (ROS). Herein, polytetrafluoroethylene (PTFE) was electrodeposited onto the surface of Sb–SnO2-doped TiO2 nanotubes (TNT/Sb–SnO2/5PTFE-300) to enhance electrochemical activity. The resulting nanoelectrode achieved removal efficiencies of 96.2 % for benzene and 97.6 % for toluene. The minimal variation in the degradation of benzene and toluene following 8 cycles of use verified its stability. Experiments confirmed that •OH was the primary ROS. Hydrophobic surface of the nanoelectrode promoted the one-electron reaction of H2O oxidation, leading to •OH concentrations that were 54.0 and 27.5 times higher, respectively, than those produced by Ti/Sb–SnO2, and 4.7 and 2.0 times higher, respectively, than those produced by TNT/Sb–SnO2-300. Moreover, intermediate products suggested the ring-opening of benzene and toluene, yielding easily degradable small organic molecules. The electrochemical oxidation (EO) process revealed a competitive relationship between benzene and toluene, with toluene exhibiting stronger competitive effects (kinetic constant was 2.1 times that of benzene). The efficient nanoelectrode provide a novel approach for the removal of benzene and toluene from aquatic environments through EO.
使用疏水性聚四氟乙烯 (PTFE) 修饰的 Sb-SnO2 掺杂 TiO2 纳米管增强水环境中苯和甲苯的电化学氧化能力
苯和甲苯在地下水中的存在对水生生态系统和人类健康都构成了严重威胁。电化学去除这些污染物的主要障碍是活性氧(ROS)生成量低。在这里,聚四氟乙烯(PTFE)被电沉积到掺杂了 Sb-SnO2 的 TiO2 纳米管(TNT/Sb-SnO2/5PTFE-300)表面,以提高电化学活性。由此产生的纳米电极对苯的去除率达到 96.2%,对甲苯的去除率达到 97.6%。在使用 8 个周期后,苯和甲苯的降解变化极小,这证明了其稳定性。实验证实,-OH 是主要的 ROS。纳米电极的疏水表面促进了 H2O 氧化的单电子反应,导致产生的 -OH 浓度分别是 Ti/Sb-SnO2 产生的 -OH 浓度的 54.0 倍和 27.5 倍,是 TNT/Sb-SnO2-300 产生的 -OH 浓度的 4.7 倍和 2.0 倍。此外,中间产物表明苯和甲苯开环,生成易降解的小有机分子。电化学氧化(EO)过程揭示了苯和甲苯之间的竞争关系,甲苯表现出更强的竞争效应(动力学常数是苯的 2.1 倍)。该高效纳米电极为通过环氧乙烷去除水生环境中的苯和甲苯提供了一种新方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.60
自引率
2.10%
发文量
2812
审稿时长
49 days
期刊介绍:
Progress in Natural Science: Materials International provides scientists and engineers throughout the world with a central vehicle for the exchange and dissemination of basic theoretical studies and applied research of advanced materials. The emphasis is placed on original research, both analytical and experimental, which is of permanent interest to engineers and scientists, covering all aspects of new materials and technologies, such as, energy and environmental materials; advanced structural materials; advanced transportation materials, functional and electronic materials; nano-scale and amorphous materials; health and biological materials; materials modeling and simulation; materials characterization; and so on. The latest research achievements and innovative papers in basic theoretical studies and applied research of material science will be carefully selected and promptly reported. Thus, the aim of this Journal is to serve the global materials science and technology community with the latest research findings.
As a service to readers, an international bibliography of recent publications in advanced materials is published bimonthly.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


