Pentafluorosulfanylation of Acrylamides: The Synthesis of SF5-Containing Isoquinolinediones with SF5Cl
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2024-10-24
DOI:10.1021/acs.joc.4c0218110.1021/acs.joc.4c02181
引用次数: 0
Abstract
We disclose herein an efficient and facile method for the synthesis of SF5-containing isoquinolinediones with an all-carbon quaternary stereocenter via intramolecular pentafluorosulfanylation of acrylamides using SF5Cl as a pentafluorosulfanylation reagent. The protocol proceeds under mild reaction conditions and enjoys a broad substrate scope, wide functional group compatibility, and high atom- and step-economy. A radical mechanism involving the SF5 radical cascade addition/cyclization of acrylamides is proposed.
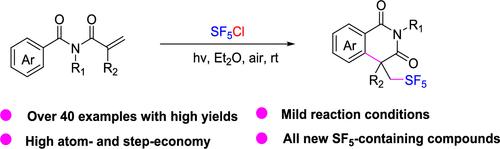
丙烯酰胺的五氟磺酰化反应:用 SF5Cl 合成含 SF5 的异quinolinediones
我们在此公开了一种高效简便的方法,该方法以 SF5Cl 为五氟磺酰化试剂,通过丙烯酰胺的分子内五氟磺酰化反应,合成具有全碳四元立体中心的含 SF5 的异喹啉二酮。该方案在温和的反应条件下进行,具有广泛的底物范围、广泛的官能团兼容性以及较高的原子和阶跃经济性。提出了一种涉及 SF5 自由基级联加成/环化丙烯酰胺的自由基机理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


