A conjugated oligoelectrolyte for the recognition of uranyl ion in aqueous and soil samples via RGB method
IF 4.3
2区 化学
Q1 SPECTROSCOPY
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy
Pub Date : 2024-10-29
DOI:10.1016/j.saa.2024.125355
引用次数: 0
Abstract
The development of selective and practically applicable sensors for monitoring trace uranyl ions (UO22+) in an aqueous medium is the biggest challenge. This study presents the development of a conjugated oligoelectrolyte-based probe (COE) for the selective detection of UO22+ ions in water bodies. The COE is a water-soluble probe having an organic backbone with two ionic pendants at the terminal points. It changes its color to a dark yellow selectively in the presence of UO22+ ions. This visible change was integrated with a smartphone RGB color quantification method. The COE displayed an RGB chemo-dosimeter to selectively monitor UO22+ ions without interference from other metal ions. In the parallel experiment, COE displays a spectrofluorimetric emission signal at λems. = 525 nm (with λexc. = 420 nm), which exhibits quenching of signal when interacted with UO22+ ions. The limit of detection (LOD) is found to be 3.07 × 10−2 µM and 4.50 µM by spectrofluorimetric and RGB color value methods, respectively. 1H NMR and XPS analysis investigated the mode of interaction, and it suggested that the quenching of the emission signal was due to the interaction between the electron-rich azomethine site of COE and UO22+ ion. The smartphone-based RGB color analysis makes COE a potential probe with reduced operation time and offers a fresh approach for the immediate, real-time detection of UO22+ ions in aqueous and soil samples.
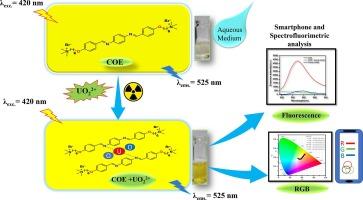
通过 RGB 法识别水和土壤样品中铀离子的共轭低聚电解质。
开发用于监测水介质中痕量铀酰离子(UO22+)的选择性和实际应用性传感器是最大的挑战。本研究介绍了一种基于共轭寡电解质的探针(COE),用于选择性检测水体中的 UO22+ 离子。COE 是一种水溶性探针,具有一个有机骨架,末端有两个离子垂体。在 UO22+ 离子存在时,它的颜色会选择性地变为深黄色。这种可见变化与智能手机的 RGB 颜色量化方法相结合。COE 显示的 RGB 化学剂量计可选择性地监测 UO22+ 离子,而不受其他金属离子的干扰。在平行实验中,COE 在 λems. = 525 nm(λexc. = 420 nm)处显示出光谱荧光发射信号,当与 UO22+ 离子相互作用时,信号会淬灭。分光荧光法和 RGB 色度法的检测限(LOD)分别为 3.07 × 10-2 µM 和 4.50 µM。1H NMR 和 XPS 分析研究了相互作用的模式,结果表明发射信号的淬灭是由于 COE 的富电子偶氮甲基位点与 UO22+ 离子之间的相互作用。基于智能手机的 RGB 颜色分析使 COE 成为一种潜在的探针,缩短了操作时间,为即时、实时检测水和土壤样品中的 UO22+ 离子提供了一种全新的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.40
自引率
11.40%
发文量
1364
审稿时长
40 days
期刊介绍:
Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy (SAA) is an interdisciplinary journal which spans from basic to applied aspects of optical spectroscopy in chemistry, medicine, biology, and materials science.
The journal publishes original scientific papers that feature high-quality spectroscopic data and analysis. From the broad range of optical spectroscopies, the emphasis is on electronic, vibrational or rotational spectra of molecules, rather than on spectroscopy based on magnetic moments.
Criteria for publication in SAA are novelty, uniqueness, and outstanding quality. Routine applications of spectroscopic techniques and computational methods are not appropriate.
Topics of particular interest of Spectrochimica Acta Part A include, but are not limited to:
Spectroscopy and dynamics of bioanalytical, biomedical, environmental, and atmospheric sciences,
Novel experimental techniques or instrumentation for molecular spectroscopy,
Novel theoretical and computational methods,
Novel applications in photochemistry and photobiology,
Novel interpretational approaches as well as advances in data analysis based on electronic or vibrational spectroscopy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


