Experimental and theoretical studies of spherical hydroxylamine hydrochloride intercalated molybdenum disulfide for the removal of U(VI), Eu(III), and Cr(VI)
IF 8.1
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
The current environmental problem is the coexistence of multiple pollutants rather than a single pollutant. In this study, U(VI), Eu(III), and Cr(VI) are selected as representatives of the actinides, lanthanide elements, and heavy metal elements for removal study. The hydroxylamine hydrochloride intercalated molybdenum disulfide (HAH/MoS2) was prepared to remove these contaminants. The insertion of hydroxylamine hydrochloride increased layer spacing, which was conducive to the pollutant molecules entering the molybdenum disulfide layer. HAH/MoS2 revealed a spherical shape with a rough surface and relatively high anti-interference. The maximum adsorption capacities of HAH/MoS2 for U(VI), Eu(III), and Cr(VI) reached 104.9 mg/g, 72.9 mg/g, and 81.4 mg/g, respectively. The adsorption mechanism of U(VI) was interlayer adsorption at pH < 6.2 and surface complexation at pH > 6.2. Similarly, the removal of Eu(III) was interlayer adsorption at pH < 5.0, interlayer adsorption and surface complexation at pH 5.0–7.7, and forming precipitation Eu(OH)3(s) at pH > 7.7. The removal of Cr(VI) depended on surface complexation at pH < 4.0 and interlayer adsorption at pH > 4.0. These ions were more likely to be adsorbed between layers instead of at the surface. Compared to U(VI) and Cr(VI), Eu(III) was more easily adsorbed at the interlamination of HAH/MoS2. From the point of view of charge transfer, U(VI) and Eu(III) tended to give away electrons, and Cr(VI) tended to gain electrons in the removal process. This work can offer a new perspective for the design and application of two-dimensional materials for multiple pollutants removal.
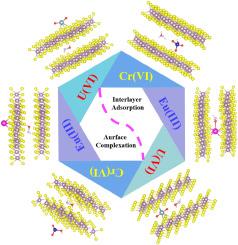
球形羟胺盐酸盐夹杂二硫化钼去除 U(VI)、Eu(III) 和 Cr(VI) 的实验和理论研究。
当前的环境问题是多种污染物并存,而非单一污染物。本研究选择 U(VI)、Eu(III) 和 Cr(VI) 作为锕系元素、镧系元素和重金属元素的代表进行去除研究。为去除这些污染物,制备了盐酸羟胺插层二硫化钼(HAH/MoS2)。盐酸羟胺的插入增加了层间距,有利于污染物分子进入二硫化钼层。HAH/MoS2 呈球形,表面粗糙,抗干扰能力相对较强。HAH/MoS2 对 U(VI)、Eu(III)和 Cr(VI) 的最大吸附容量分别达到 104.9 mg/g、72.9 mg/g 和 81.4 mg/g。在 pH 值小于 6.2 时,U(VI) 的吸附机理是层间吸附,而在 pH 值大于 6.2 时,则是表面络合。同样,Eu(III)的去除机理在 pH 值小于 5.0 时为层间吸附,在 pH 值为 5.0-7.7 时为层间吸附和表面络合,在 pH 值大于 7.7 时形成沉淀 Eu(OH)3(s)。六价铬的去除取决于 pH < 4.0 时的表面络合和 pH > 4.0 时的层间吸附。这些离子更有可能被吸附在层间而不是表面。与铀(VI)和铬(VI)相比,Eu(III)更容易吸附在 HAH/MoS2 的层间。从电荷转移的角度来看,在去除过程中,U(VI)和Eu(III)倾向于放出电子,而Cr(VI)倾向于获得电子。这项研究为设计和应用二维材料去除多种污染物提供了新的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemosphere
环境科学-环境科学
CiteScore
15.80
自引率
8.00%
发文量
4975
审稿时长
3.4 months
期刊介绍:
Chemosphere, being an international multidisciplinary journal, is dedicated to publishing original communications and review articles on chemicals in the environment. The scope covers a wide range of topics, including the identification, quantification, behavior, fate, toxicology, treatment, and remediation of chemicals in the bio-, hydro-, litho-, and atmosphere, ensuring the broad dissemination of research in this field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


