Impact of sleep restriction on biomarkers of thyroid function: Two pooled randomized trials
IF 3.8
2区 医学
Q1 CLINICAL NEUROLOGY
引用次数: 0
Abstract
Background
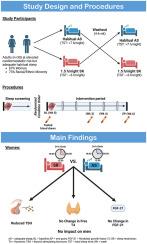
Chronic, mildly insufficient sleep is associated with increased cardiometabolic risk, but whether the regulation of thyroid hormones and related growth factors are mechanisms of this association is unclear. We investigated whether 6 wk of mild sleep restriction (SR) alters levels of free thyroxine (FT4), thyroid stimulating hormone (TSH), and fibroblast growth factor-21 (FGF-21), a modulator of FT4, in adults with adequate habitual sleep (AS; 7–9 h/night).
Methods
Healthy adults participated in one of two randomized, crossover studies with identical 6-wk intervention phases: AS and SR (1.5 h/night < AS). Fasted blood samples were collected at baseline and endpoint of each phase. Outcomes were concentrations of FT4, TSH, and FGF-21 (women only). Linear mixed models tested the effects of SR vs AS on the outcomes, adjusting for baseline levels, week, sex, and sex-by-condition interaction.
Results
Thirty participants (20 women; 73% racial/ethnic minority; age 21–64 y [M±SD = 36.2 ± 12.8 y]) were included. In the full sample, no effects of SR on FT4 (β±SE = 0.02 ± 0.04, p = 0.654) or TSH (β±SE = −0.02 ± 0.04, p = 0.650) were observed; however, there were sex-by-condition interactions for both FT4 (p-interaction = 0.056) and TSH (p-interaction = 0.049). In sex-stratified analyses, TSH was reduced in SR vs. AS in women (β±SE = −0.11 ± 0.04, p = 0.011, Cohen's f2 = 0.55) but not men (β±SE = 0.09 ± 0.08, p = 0.261). Among women (n = 17), FGF-21 was not significantly different between conditions (β±SE = 8.51 ± 17.70, p = 0.638).
Conclusion
Prolonged mild SR reduces TSH in women, whereas FT4 and FGF-21 remain unaffected compared with AS. If sustained, disruptions to the thyrotropic axis in women may contribute to their more pronounced cardiometabolic risk in response to SR compared with men.
限制睡眠对甲状腺功能生物标志物的影响:两项合并随机试验
背景:长期轻度睡眠不足与心脏代谢风险增加有关,但甲状腺激素和相关生长因子的调节是否是这种关联的机制尚不清楚。我们研究了 6 周轻度睡眠限制(SR)是否会改变具有充足习惯性睡眠(AS;7-9 小时/晚)的成年人体内游离甲状腺素(FT4)、促甲状腺激素(TSH)和成纤维细胞生长因子-21(FGF-21)的水平:AS 和 SR(1.5 小时/晚):共纳入 30 名参与者(20 名女性;73% 为少数民族;年龄 21-64 岁 [M±SD = 36.2 ± 12.8 岁])。在全样本中,未观察到 SR 对 FT4(β±SE = 0.02 ± 0.04,p = 0.654)或 TSH(β±SE = -0.02 ± 0.04,p = 0.650)的影响;但是,FT4(p-交互作用 = 0.056)和 TSH(p-交互作用 = 0.049)存在性别-条件交互作用。在性别分层分析中,女性(β±SE = -0.11 ± 0.04,p = 0.011,Cohen's f2 = 0.55)SR 与 AS 相比 TSH 降低,但男性(β±SE = 0.09 ± 0.08,p = 0.261)不降反升。在女性(n = 17)中,FGF-21在不同条件下没有显著差异(β±SE = 8.51 ± 17.70,p = 0.638):结论:长期轻度SR会降低女性促甲状腺激素(TSH),而FT4和FGF-21与AS相比不受影响。如果持续下去,女性甲状腺轴的紊乱可能会导致她们在SR反应中比男性更明显的心脏代谢风险。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Sleep medicine
医学-临床神经学
CiteScore
8.40
自引率
6.20%
发文量
1060
审稿时长
49 days
期刊介绍:
Sleep Medicine aims to be a journal no one involved in clinical sleep medicine can do without.
A journal primarily focussing on the human aspects of sleep, integrating the various disciplines that are involved in sleep medicine: neurology, clinical neurophysiology, internal medicine (particularly pulmonology and cardiology), psychology, psychiatry, sleep technology, pediatrics, neurosurgery, otorhinolaryngology, and dentistry.
The journal publishes the following types of articles: Reviews (also intended as a way to bridge the gap between basic sleep research and clinical relevance); Original Research Articles; Full-length articles; Brief communications; Controversies; Case reports; Letters to the Editor; Journal search and commentaries; Book reviews; Meeting announcements; Listing of relevant organisations plus web sites.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


