Leveraging machine learning to streamline the development of liposomal drug delivery systems
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Drug delivery systems efficiently and safely administer therapeutic agents to specific body sites. Liposomes, spherical vesicles made of phospholipid bilayers, have become a powerful tool in this field, especially with the rise of microfluidic manufacturing during the COVID-19 pandemic. Despite its efficiency, microfluidic liposomal production poses challenges, often requiring laborious, optimization on a case-by-case basis. This is due to a lack of comprehensive understanding and robust methodologies, compounded by limited data on microfluidic production with varying lipids. Artificial intelligence offers promise in predicting lipid behaviour during microfluidic production, with the still unexploited potential of streamlining development. Herein we employ machine learning to predict critical quality attributes and process parameters for microfluidic-based liposome production. Validated models predict liposome formation, size, and production parameters, significantly advancing our understanding of lipid behaviour. Extensive model analysis enhanced interpretability and investigated underlying mechanisms, supporting the transition to microfluidic production. Unlocking the potential of machine learning in drug development can accelerate pharmaceutical innovation, making drug delivery systems more adaptable and accessible.
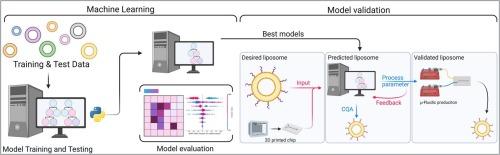
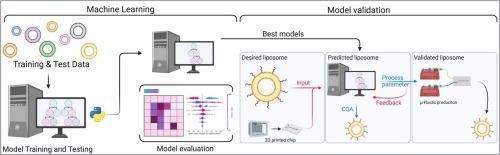
利用机器学习简化脂质体给药系统的开发。
药物输送系统能高效、安全地将治疗药物输送到人体特定部位。脂质体是由磷脂双分子层构成的球形囊泡,已成为这一领域的有力工具,尤其是在 COVID-19 大流行期间,微流控生产技术的兴起更是如此。尽管微流控脂质体生产效率很高,但它也带来了挑战,往往需要逐个进行费力的优化。这是由于缺乏全面的了解和可靠的方法,再加上使用不同脂质进行微流控生产的数据有限。人工智能在预测微流控生产过程中的脂质行为方面大有可为,其简化开发的潜力仍有待开发。在此,我们利用机器学习预测基于微流控生产脂质体的关键质量属性和工艺参数。经过验证的模型可以预测脂质体的形成、大小和生产参数,极大地促进了我们对脂质行为的理解。广泛的模型分析增强了可解释性并研究了潜在机制,为过渡到微流控生产提供了支持。释放机器学习在药物开发中的潜力可以加速制药创新,使药物输送系统更具适应性和可及性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


