Central role of squid gene during oocyte development in the Hemiptera Rhodnius prolixus
IF 2.3
2区 农林科学
Q1 ENTOMOLOGY
引用次数: 0
Abstract
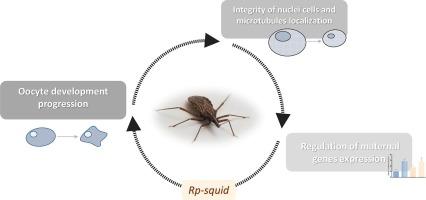
Oocyte polarity establishment is a conserved and crucial phenomenon for embryonic development. It relies on the precise spatial localization of maternal factors deposited during oocyte development, which is essential for establishing and maintaining cell polarity and subsequently specifying embryonic axes. The heterogeneous nuclear ribonucleoprotein (hnRNP) encoded by the squid (sqd) gene has been implicated in mRNA localization and embryonic axis establishment in Drosophila melanogaster. Comparative genomics allowed for the identification of a homologue in Rhodnius prolixus. In this study, we investigated the function of Rp-sqd during oogenesis and early embryonic development. We observed persistent expression of Rp-sqd during oocyte development, with localization in the cytoplasm of ovary germarium and growing oocytes in previtellogenic and vitellogenic stages. A Parental RNA interference (RNAi) experiment targeting Rp-sqd resulted in female sterility. The ovaries showed disrupted oocyte development, disarray of follicular epithelium, and affected nurse cells integrity. Immunostaining and microscopic techniques revealed microtubule disarray and a reduction in the presence of organelles in the trophic cords that connect the germarium with the oocytes. The Rp-sqd depletion impacted the transcript expression of maternal mRNAs involved in apoptosis, axis formation, oogenesis, and cytoskeleton maintenance, indicating a pleiotropic function of Rp-sqd during oogenesis. This study provides new insights into the genetic basis of R. prolixus oogenesis, highlighting the crucial role of Rp-sqd in oocyte development, fertility, and germarium integrity. These findings contribute to our understanding of insect developmental processes, provide a foundation for future investigations into reproduction, and reveal the regulatory mechanisms governing the process.
乌贼基因在Rhodnius prolixus半翅目昆虫卵母细胞发育过程中的核心作用
卵母细胞极性的建立是胚胎发育过程中一个重要的保守现象。它依赖于卵母细胞发育过程中沉积的母体因子的精确空间定位,这对于建立和维持细胞极性以及随后指定胚胎轴至关重要。乌贼(sqd)基因编码的异质核核糖核蛋白(hnRNP)与黑腹果蝇的 mRNA 定位和胚胎轴建立有关。比较基因组学发现了 Rhodnius prolixus 的同源物。在本研究中,我们研究了 Rp-sqd 在卵子发生和早期胚胎发育过程中的功能。我们观察到 Rp-sqd 在卵母细胞发育过程中持续表达,定位于卵巢胚芽和卵黄发生前及卵黄发生期生长卵母细胞的细胞质中。针对 Rp-sqd 的亲本 RNA 干扰(RNAi)实验导致雌性不育。卵巢中的卵母细胞发育受到破坏,卵泡上皮细胞杂乱无章,滋养细胞的完整性也受到影响。免疫染色和显微镜技术显示,微管混乱,连接生殖细胞和卵母细胞的营养索中的细胞器减少。Rp-sqd的缺失影响了参与细胞凋亡、轴形成、卵子发生和细胞骨架维持的母体mRNA的转录表达,表明Rp-sqd在卵子发生过程中具有多重功能。这项研究为我们揭示 R. prolixus 卵子发生的遗传基础提供了新的视角,凸显了 Rp-sqd 在卵母细胞发育、生殖力和生殖室完整性中的关键作用。这些发现有助于我们了解昆虫的发育过程,为今后的繁殖研究奠定了基础,并揭示了这一过程的调控机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of insect physiology
生物-昆虫学
CiteScore
4.50
自引率
4.50%
发文量
77
审稿时长
57 days
期刊介绍:
All aspects of insect physiology are published in this journal which will also accept papers on the physiology of other arthropods, if the referees consider the work to be of general interest. The coverage includes endocrinology (in relation to moulting, reproduction and metabolism), pheromones, neurobiology (cellular, integrative and developmental), physiological pharmacology, nutrition (food selection, digestion and absorption), homeostasis, excretion, reproduction and behaviour. Papers covering functional genomics and molecular approaches to physiological problems will also be included. Communications on structure and applied entomology can be published if the subject matter has an explicit bearing on the physiology of arthropods. Review articles and novel method papers are also welcomed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


