NIR-activated Janus nanomotors with promoted tumor permeability for synergistic photo-immunotherapy
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Nanoparticle-based photo-immunotherapy has become an attractive strategy to eliminate tumors and activate host immune responses. However, the therapeutic efficacy is heavily restricted by low tumoral penetration and immunosuppressive tumor microenvironment (TME). Herein, near infrared laser (NIR)-propelled Janus nanomotors were presented for deep tumoral penetration, photothermal tumor ablation and photothermal-triggered augmented immunotherapy. The Janus nanomotors (AuNR/PMO@CPG) were constructed with gold nanorods (AuNR) and periodic mesoporous organo-silica nanospheres (PMO), followed by loading of immune adjuvant (CPG ODNs). Under NIR irradiation, the nanomotors exhibited superior photothermal effect, which produced active motion with a speed of 19.3 µm/s for deep tumor penetration and accumulation in vivo. Moreover, the good photothermal heating also benefited effective photothermal ablation to trigger immunogenic cell death (ICD). Subsequently, the ICD effect promoted the release of tumor-associated antigens (TAAs) and damage associated molecular patterns (DAMPs), and further generated abundant tumor vaccines in situ for reprograming the immunosuppressive TME in combination with CPG ODNs to inhibit tumor growth. As a result, a notable in vivo synergistic therapeutic effect was realized on CT26-bearing mice by combining photothermal therapy-induced ICD with modulation of immunosuppressive TME. Thus, we believe that the synthesized nanomotors can provide a new inspect to boost photothermal therapy-induced ICD in tumor immunotherapy.
Statement of Significance
Nanoparticle-based synergistic photo-immunotherapy has become a popular strategy to eliminate tumors and activate host immune responses. However, the therapeutic efficacy is heavily restricted by low tumoral penetration and immunosuppressive tumor microenvironment (TME). In this work, near infrared laser (NIR)-propelled Janus nanomotors were presented for deep tumoral penetration, photothermal tumor ablation and photothermal-triggered augmented immunotherapy. Under NIR irradiation, the nanomotors exhibited a superior photothermal effect, which produced active motion for deep tumor penetration and accumulation in vivo. Moreover, good photothermal heating also facilitated effective photothermal ablation to trigger immunogenic cell death (ICD), which promoted the release of tumor-associated antigens and damage-associated molecular patterns (DAMPs), and further generated abundant tumor vaccines in situ for reprograming the immunosuppressive TME to inhibit tumor growth.
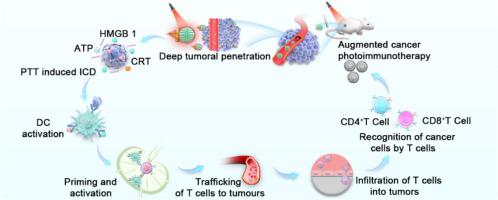
促进肿瘤通透性的近红外激活 Janus 纳米马达可用于协同光免疫疗法。
基于纳米粒子的光免疫疗法已成为消除肿瘤和激活宿主免疫反应的一种极具吸引力的策略。然而,由于肿瘤穿透力低和肿瘤微环境(TME)具有免疫抑制作用,治疗效果受到严重限制。本文介绍了由近红外激光(NIR)推动的Janus纳米马达,用于深度穿透肿瘤、光热消融肿瘤和光热触发的增强免疫疗法。Janus纳米马达(AuNR/PMO@CPG JNMs)由金纳米棒(AuNR)和周期性介孔有机硅纳米球(PMO)构建而成,然后加入免疫佐剂(CPG ODNs)。在近红外照射下,纳米马达表现出卓越的光热效应,能以每秒 19.3 微米的速度产生主动运动,从而深入肿瘤并在体内蓄积。此外,良好的光热加热还有利于有效的光热消融,从而引发免疫性细胞死亡(ICD)。随后,ICD效应促进了肿瘤相关抗原(TAAs)和损伤相关分子模式(DAMPs)的释放,并进一步在原位生成丰富的肿瘤疫苗,与CPG ODNs结合重编程免疫抑制TME,抑制肿瘤生长。结果,通过将光热疗法诱导的 ICD 与免疫抑制性 TME 的调节相结合,在 CT26 小鼠身上实现了显著的体内协同治疗效果。因此,我们认为合成的纳米马达可以为光热疗法诱导的 ICD 在肿瘤免疫疗法中的应用提供新的途径。意义说明:基于纳米粒子的协同光免疫疗法已成为消除肿瘤和激活宿主免疫反应的流行策略。然而,由于肿瘤穿透力低和肿瘤微环境(TME)具有免疫抑制作用,其疗效受到很大限制。本研究提出了近红外激光(NIR)推动的 Janus 纳米马达,用于深度穿透肿瘤、光热消融肿瘤和光热触发增强免疫疗法。在近红外辐照下,纳米马达表现出卓越的光热效应,能在体内产生主动运动,实现肿瘤的深度穿透和蓄积。此外,良好的光热加热还有助于有效的光热消融,从而触发免疫原性细胞死亡(ICD),促进肿瘤相关抗原和损伤相关分子模式(DAMPs)的释放,并进一步在原位生成丰富的肿瘤疫苗,对免疫抑制性TME进行重编程,抑制肿瘤生长。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


