Enhancing RNA encapsulation quantification in lipid nanoparticles: Sustainable alternatives to Triton X-100 in the RiboGreen assay
IF 4.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2024-10-28
DOI:10.1016/j.ejpb.2024.114571
引用次数: 0
Abstract
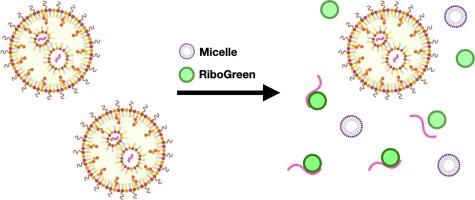
To quantify concentration and encapsulation efficiency (EE) of mRNA in lipid nanoparticles (LNPs) the RiboGreen assay is extensively used. As part of this assay, a surfactant is used to release mRNA from LNPs for detection with the RiboGreen dye. So far, the surfactant of choice has been Triton X-100, which is harmful to human health and the environment. Alternatives to Triton X-100 are therefore needed, but surprisingly no such effort has yet been described in the literature. Here we show how three, less harmful, surfactants (Brij 93, Zwittergent 3–14 and Tween 20) compare to Triton X-100 for releasing mRNA from LNPs for detection with the RiboGreen assay. We found that Zwittergent 3–14 and Tween 20 at high concentrations (0.5 %) are at the minimum as effective as Triton X-100 at high concentration (0.5 %) across three different mRNA-LNP formulations. Interestingly, Tween 20 was the most effective at releasing mRNA from LNPs, across all concentration ranges explored (0.0025 %, 0.01 %, 0.1 % and to 0.5 % (v/v)) highlighting its potency at solubilizing the three different LNP formulations. Our results show that Tween 20 can be used as an alternative to Triton X-100 in the RiboGreen assay, resulting in more accurate quantification of the total mRNA concentration and EE%, as well as making the assay more environmentally friendly. Such improvement could potentially increase the likelihood of identifying therapeutically attractive hard-to-solubilize LNP-mRNA formulations that would be discharged when using Triton X-100 due to their apparent low EE values, as well as ensure more accurate mRNA dosing in both in vitro and in vivo studies.
增强脂质纳米颗粒中的 RNA 封装定量:RiboGreen 试验中 Triton X-100 的可持续替代品。
为了量化脂质纳米粒子(LNPs)中 mRNA 的浓度和封装效率(EE),RiboGreen 分析法被广泛使用。作为该检测方法的一部分,表面活性剂被用来从 LNPs 中释放 mRNA,以便用 RiboGreen 染料进行检测。迄今为止,首选的表面活性剂是 Triton X-100,它对人体健康和环境有害。因此,我们需要 Triton X-100 的替代品,但令人惊讶的是,文献中还没有这方面的描述。在这里,我们展示了三种危害较小的表面活性剂(Brij 93、Zwittergent 3-14 和 Tween 20)与 Triton X-100 相比,如何从 LNPs 中释放 mRNA,以便用 RiboGreen 检测法进行检测。我们发现,在三种不同的 mRNA-LNP 配方中,高浓度(0.5%)的 Zwittergent 3-14 和 Tween 20 与高浓度(0.5%)的 Triton X-100 的效果相当。有趣的是,在所探讨的所有浓度范围(0.0025%、0.01%、0.1% 至 0.5%(v/v))内,吐温 20 从 LNPs 中释放 mRNA 的效果最好,这凸显了它对三种不同 LNP 配方的增溶作用。我们的研究结果表明,在 RiboGreen 检测中,吐温 20 可替代 Triton X-100,从而更准确地量化总 mRNA 浓度和 EE%,并使检测更环保。这种改进有可能提高识别具有治疗吸引力的难以溶解的 LNP-mRNA 制剂的可能性,这些制剂在使用 Triton X-100 时会因其明显的低 EE 值而被排出,同时还能确保在体外和体内研究中的 mRNA 剂量更准确。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


