Uterine first-pass effect: Unlocking the potential of vaginally administered ritodrine-loaded thermosensitive gel for uterine drug delivery
IF 4.3
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Preterm birth (PTB) remains a leading cause of infant mortality and morbidity, significantly affecting the long-term health, welfare, and development of newborns. Tocolytics, such as ritodrine, a β2-adrenergic receptor agonist, are widely used in developing countries due to their affordability for preventing PTB by inhibiting uterine contractions. However, ritodrine's short half-life necessitates frequent administration, and prolonged high-dose usage often leads to serious maternal side effects, prompting discontinuation. The uterine first-pass effect, where vaginally administered drugs preferentially target the uterus, can enhance drug concentration in uterine tissue while minimizing systemic absorption and side effects. This study designed a kind of ritodrine-loaded thermosensitive gel (Gel@Rit) to intervene in PTB by exploiting the uterine first-pass effect and investigate its underlying mechanisms. The gel, formulated with poloxamer, demonstrated excellent temperature sensitivity and viscosity, ensuring sustained ritodrine release in vitro. Plasma pharmacokinetic and tissue distribution studies in pregnant mice confirmed the uterine first-pass effect, showing significantly higher drug concentrations in the uterus and markedly lower plasma levels following Gel@Rit administration. The distinctive drug-time curve in Gel@Rit-treated mice, along with uterine tissue fluorescence profiles, elucidated four mechanisms of uterine localization: diffusion through reproductive tract cavities, penetration via vaginal and uterine structures, diffusion through systemic circulation, and retrograde transvaginal veno-uterine artery exchange. This study provides valuable insights into vaginal drug delivery research methodologies, advancing therapeutic strategies for uterine-related conditions and benefiting clinical outcomes in PTB prevention.
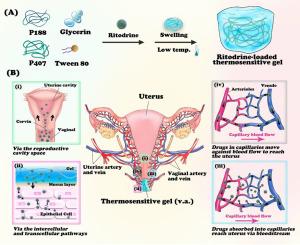
子宫首过效应:挖掘经阴道给药的利托君热敏凝胶在子宫给药方面的潜力。
早产(PTB)仍然是婴儿死亡和发病的主要原因,严重影响新生儿的长期健康、福利和发育。由于价格低廉,β2-肾上腺素能受体激动剂利托君等催产药在发展中国家被广泛使用,通过抑制子宫收缩来预防早产。然而,利托君的半衰期较短,需要频繁给药,长期大剂量使用往往会导致严重的孕产妇副作用,从而导致停药。子宫首过效应(阴道给药优先针对子宫)可提高药物在子宫组织中的浓度,同时减少全身吸收和副作用。本研究设计了一种利托君热敏凝胶(Gel@Rit),利用子宫首过效应干预PTB,并研究其潜在机制。该凝胶由聚氧乙烯醚配制而成,具有出色的温度敏感性和粘度,可确保利托君在体外持续释放。在妊娠小鼠体内进行的血浆药代动力学和组织分布研究证实了子宫首过效应,结果表明,服用 Gel@Rit 后,子宫内的药物浓度显著升高,而血浆中的药物浓度则明显降低。经 Gel@Rit 处理的小鼠体内独特的药物时间曲线以及子宫组织荧光曲线阐明了子宫定位的四种机制:通过生殖道腔扩散、通过阴道和子宫结构渗透、通过全身循环扩散以及逆行经阴道静脉-子宫动脉交换。这项研究为阴道给药研究方法提供了宝贵的见解,推动了子宫相关疾病的治疗策略,并有利于预防宫颈息肉的临床治疗效果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


