Site-specific dimerization of interleukin-11 alleviates bleomycin-induced pulmonary fibrosis in mice
IF 4.3
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Interleukin-11 (IL-11) has recently been identified as a critical profibrotic cytokine, and IL-11 signaling pathway via IL-11Rα and GP130 receptors has been shown to be a promising therapeutic target for the treatment of fibrotic diseases. Herein, we devised two kinds of IL-11 dimer with receptor-biased binding ability through site-specific crosslinking at the interface involving GP130 binding and signaling, aiming to explore their therapeutic potentials for bleomycin-induced pulmonary fibrosis in mice. A single cysteine mutation at site W147 of human IL-11 (IL-11 W147C) was conducted for site-specific crosslinking. The ability of GP130 to bind to IL-11 W147C dimer was substantially weakened by cysteine-based dimerization, while the ability of IL-11 W147C dimer to bind to IL-11Rα was almost entirely preserved or even enhanced. The IL-11 W147C dimer potently inhibited TF-1 cell proliferation and TGF-β1-induced human lung fibroblast differentiation into myofibroblasts. We also showed that dimerization substantially extended the circulation time of IL-11 W147C dimer in healthy rats. Subcutaneous administration of IL-11 W147C dimer significantly reduced extracellular matrix deposition, preserved alveolar architecture and alleviated pulmonary fibrosis development in mice. The findings of this study may provide a general strategy for the design of cytokine-based receptor-biased antagonists and agonists targeting these multifaceted signaling pathways.
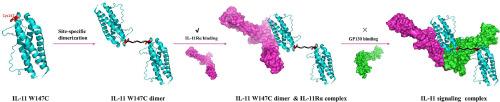
白细胞介素-11的位点特异性二聚化可减轻博莱霉素诱导的小鼠肺纤维化。
白细胞介素-11(IL-11)最近被确认为一种关键的促纤维化细胞因子,而通过IL-11Rα和GP130受体的IL-11信号通路已被证明是治疗纤维化疾病的一个有前景的治疗靶点。在此,我们通过在涉及GP130结合和信号传导的界面上进行位点特异性交联,设计了两种具有受体偏向结合能力的IL-11二聚体,旨在探索它们对博莱霉素诱导的小鼠肺纤维化的治疗潜力。研究人员对人IL-11的W147位点(IL-11 W147C)进行了单半胱氨酸突变,以实现位点特异性交联。基于半胱氨酸的二聚化大大削弱了GP130与IL-11 W147C二聚体的结合能力,而IL-11 W147C二聚体与IL-11Rα的结合能力几乎完全保留甚至增强。IL-11 W147C二聚体能有效抑制TF-1细胞增殖和TGF-β1诱导的人肺成纤维细胞向肌成纤维细胞分化。我们还发现,二聚化大大延长了 IL-11 W147C 二聚体在健康大鼠体内的循环时间。IL-11 W147C 二聚体皮下注射可显著减少细胞外基质沉积,保护肺泡结构,缓解小鼠肺纤维化的发展。这项研究的发现可能为设计基于细胞因子受体的拮抗剂和激动剂提供了一种针对这些多方面信号通路的通用策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


