Negative feedback regulation of GLABRA1 contributes to epidermal cell patterning in the Arabidopsis root
IF 2.5
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochemical and biophysical research communications
Pub Date : 2024-10-26
DOI:10.1016/j.bbrc.2024.150869
引用次数: 0
Abstract
GLABRA1 (GL1), which encodes an R2R3 MYB transcription factor, is a key regulator of trichome patterning in the aerial organs of Arabidopsis (Arabidopsis thaliana). Although it has been generally assumed that GL1 functions exclusively in shoots and is not expressed in roots, reverse transcription polymerase chain reaction (RT-PCR) analysis has revealed that GL1 is indeed expressed in roots. To investigate whether GL1 plays a role in root epidermal patterning, we analyzed the effects of gl1 mutations in sensitized genetic backgrounds. Our findings show that gl1 mutants enhance the root epidermal phenotype of a weak allele of the werewolf (wer) mutant and suppress the phenotype of the caprice (cpc) mutant. We also demonstrate that the GL1 promoter is active in N-position epidermal cells, and that the GFP-GL1 fusion protein is predominantly localized in the nucleus of N-position cells. Furthermore, we provide evidence that GL1 expression is positively regulated by WER, GLABRA3, ENHANCER OF GLABRA3, and TRANSPARENT TESTA GLABRA1, while negatively regulated by CPC, TRIPTYCHON, and GLABRA2 (GL2). Notably, GL2, which is positively regulated by GL1, moderately represses GL1 expression, and both GL1 and GL2 are positively regulated by WER in N-position cells. These findings suggest that a negative feedback regulation of GL1 expression via GL2 contributes to the fine-tuning of non-hair cell fate determination in Arabidopsis root epidermis.
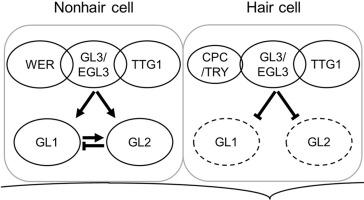
GLABRA1 的负反馈调节有助于拟南芥根部表皮细胞模式化。
GLABRA1(GL1)编码一个 R2R3 MYB 转录因子,是拟南芥(Arabidopsis thaliana)气生器官中毛状体形态的关键调节因子。尽管人们普遍认为 GL1 只在芽中发挥作用,而不在根中表达,但反转录聚合酶链反应(RT-PCR)分析表明,GL1 确实在根中表达。为了研究 GL1 是否在根表皮模式化中发挥作用,我们分析了在敏化遗传背景下 gl1 突变的影响。我们的研究结果表明,gl1突变体增强了狼獾(wer)突变体弱等位基因的根表皮表型,并抑制了caprice(cpc)突变体的表型。我们还证明 GL1 启动子在 N 位表皮细胞中具有活性,GFP-GL1 融合蛋白主要定位于 N 位细胞的细胞核中。此外,我们还提供了证据,证明 GL1 的表达受 WER、GLABRA3、ENHANCER OF GLABRA3 和 TRANSPARENT TESTA GLABRA1 的正调控,而受 CPC、TRIPTYCHON 和 GLABRA2(GL2)的负调控。值得注意的是,受 GL1 正向调节的 GL2 可适度抑制 GL1 的表达,而在 N 位细胞中,GL1 和 GL2 均受 WER 的正向调节。这些发现表明,通过 GL2 对 GL1 表达的负反馈调节有助于拟南芥根表皮中非毛细胞命运决定的微调。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.10
自引率
0.00%
发文量
1400
审稿时长
14 days
期刊介绍:
Biochemical and Biophysical Research Communications is the premier international journal devoted to the very rapid dissemination of timely and significant experimental results in diverse fields of biological research. The development of the "Breakthroughs and Views" section brings the minireview format to the journal, and issues often contain collections of special interest manuscripts. BBRC is published weekly (52 issues/year).Research Areas now include: Biochemistry; biophysics; cell biology; developmental biology; immunology
; molecular biology; neurobiology; plant biology and proteomics

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


