Formulation and optimization of chitosan-based amorphous fenbendazole microparticles through a design of experiment approach
IF 5.2
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Fenbendazole is a broad-spectrum anthelmintic used in veterinary medicine. It is a lipophilic benzimidazole derivative with low water solubility (<0.1 g/L) recently studied for repositioning in cancer treatment. These potential new uses highlight the need for new dosage forms. Thus, chitosan-crosslinked microparticles were prepared by spray drying, applying a Design of Experiments approach to optimize the composition of the microparticles, evaluating the type and mass of chitosan and crosslinking agent, alongside crosslinking reaction time. The recovered optimized microparticles were characterized by infrared spectroscopy, and changes in the drug crystalline phase were studied by differential scanning calorimetry and X-ray powder diffraction, further confirmed by wide-angle X-ray scattering. After encapsulation of fenbendazole in the chitosan-crosslinked matrix, the resulting microparticles had a particle size of 2.43 μm with a polydispersion index of 0.754 and a Zeta potential value of + 49.85 mV. In vitro dissolution showed that the optimized microparticles had an improved dissolution profile compared to the non-encapsulated drug. The analysis of the encapsulated drug in the solid state showed a remarkable reduction of its crystalline properties. In conclusion, these results demonstrate that fenbendazole encapsulation into an optimized chitosan-crosslinked matrix leads to better biopharmaceutical performance.
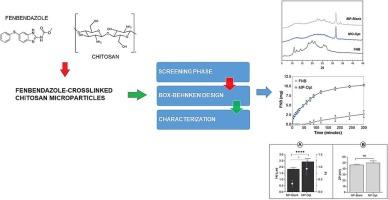
通过实验设计方法配制和优化基于壳聚糖的无定形芬苯达唑微颗粒。
芬苯达唑是一种用于兽医的广谱抗蠕虫药。它是一种亲脂性苯并咪唑衍生物,水溶性低(0.1%-0.5%)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


