Astrocytic Gap Junctions protein Cx43/Cx30 modulate EAAT1 and glutamate to mediate cerebral ischemia–reperfusion injury
IF 2.7
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
Abstract
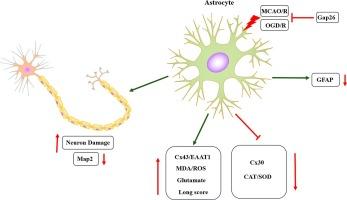
The gap connexins of astrocytes play a crucial role in facilitating neuronal coordination and maintaining the homeostasis of the central nervous system. Cx30/Cx43 are the main proteins constituting these gap junctions, and the glutamate transporter EAAT1 associates with nerve injury. However, the role and mechanism underlying the changes of astrocytic connexins and EAAT1 during cerebral ischemia–reperfusion injury remain unclear. In this study, we investigated the expressions of Cx30, Cx43, and EAAT1 in OGD/R-treated astrocytes and in a MCAO/R animal model using gap junction inhibitors and siRNAs targeting Cx43 and Cx30. The differences of cell viability, malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), reactive oxygen species (ROS) and glutamate in cells and tissues were detected. Our results indicate that OGD/R exposure leads to the decline of astrocyte activity, which, in turn, adversely affects neuronal health. Ischemia-reperfusion induced increasing Cx43 and EAAT1 expression and decreasing Cx30 expression in astrocytes and animal brain tissue. Moreover, ischemia–reperfusion resulted in heightened MDA and ROS levels and reduced CAT and SOD activities in both astrocytes and the surrounding brain tissue. The release of glutamate from astrocytes and its concentration in animal brain tissue significantly increased following ischemia–reperfusion. Inhibition Cx43 expression through Gap26 or siRNA effectively mitigated the increase in EAAT1 and glutamate levels, as well as the oxidative stress changes induced by ischemia–reperfusion. Therefore, Brain astrocytes may mediate the effects of cerebral ischemia–reperfusion injury by influencing glutamate transporters and glutamate dynamics in response to oxidative stress through Cx30/Cx43.
星形胶质细胞间隙连接蛋白Cx43/Cx30调节EAAT1和谷氨酸,介导脑缺血再灌注损伤
星形胶质细胞的间隙连接蛋白在促进神经元协调和维持中枢神经系统平衡方面发挥着至关重要的作用。Cx30/Cx43 是构成这些间隙连接的主要蛋白,谷氨酸转运体 EAAT1 与神经损伤有关。然而,脑缺血再灌注损伤期间星形胶质细胞连接蛋白和 EAAT1 变化的作用和机制仍不清楚。在这项研究中,我们使用间隙连接抑制剂和靶向 Cx43 和 Cx30 的 siRNA 研究了 OGD/R 处理的星形胶质细胞和 MCAO/R 动物模型中 Cx30、Cx43 和 EAAT1 的表达。我们检测了细胞和组织中细胞活力、丙二醛(MDA)、超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、活性氧(ROS)和谷氨酸的差异。我们的研究结果表明,OGD/R 暴露会导致星形胶质细胞活性下降,进而对神经元健康产生不利影响。缺血再灌注诱导星形胶质细胞和动物脑组织中的 Cx43 和 EAAT1 表达增加,Cx30 表达减少。此外,缺血再灌注导致星形胶质细胞和周围脑组织中的 MDA 和 ROS 水平升高,CAT 和 SOD 活性降低。缺血再灌注后,星形胶质细胞释放的谷氨酸及其在动物脑组织中的浓度显著增加。通过 Gap26 或 siRNA 抑制 Cx43 的表达能有效缓解 EAAT1 和谷氨酸水平的增加,以及缺血再灌注引起的氧化应激变化。因此,脑星形胶质细胞可能通过Cx30/Cx43影响谷氨酸转运体和谷氨酸的动态变化以应对氧化应激,从而介导脑缺血再灌注损伤的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


