α-Nucleophilic Addition to α,β-Unsaturated Carbonyl Compounds via Photocatalytically Generated α-Carbonyl Carbocations.
IF 16.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
We report the photocatalytic oxidation of α-carbonyl radicals of amides or esters to the corresponding α-carbonyl carbocations through super photoreductant CBZ6 induced redox-neutral photocatalysis. The α-carbonyl radicals are formed by the β-addition of alkyl radicals generated in situ by the photocatalytic fragmentation of N-hydroxyphthalimide esters to the α,β-unsaturated amides and esters. This method enables the α-nucleophilic addition of hydroxyl or alkoxyl radicals to amides and esters without any prefunctionalization.
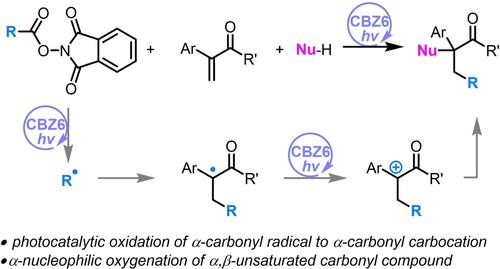
通过光催化生成的 α-Carbonyl Carbocations 对 α,β-不饱和羰基化合物进行 α-亲核加成。
我们报告了通过超级光诱导剂 CBZ6 诱导的氧化还原中性光催化作用,将酰胺或酯的 α-羰基自由基光催化氧化为相应的 α-羰基碳化物的过程。N- 羟基邻苯二甲酰亚胺酯与 α、β- 不饱和酰胺和酯的光催化碎片原位生成的烷基自由基通过 β-加成形成 α-羰基自由基。这种方法可将羟基或烷氧基自由基与酰胺和酯进行α-亲核加成,而无需任何预官能化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
26.60
自引率
6.60%
发文量
3549
审稿时长
1.5 months
期刊介绍:
Angewandte Chemie, a journal of the German Chemical Society (GDCh), maintains a leading position among scholarly journals in general chemistry with an impressive Impact Factor of 16.6 (2022 Journal Citation Reports, Clarivate, 2023). Published weekly in a reader-friendly format, it features new articles almost every day. Established in 1887, Angewandte Chemie is a prominent chemistry journal, offering a dynamic blend of Review-type articles, Highlights, Communications, and Research Articles on a weekly basis, making it unique in the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


