Advancements in π-conjugated polymers: harnessing cycloalkyl straps for high-performance π-conjugated materials
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Pendant alkyl chains are widely used to successfully obtain a wide variety of soluble linear 1D π-conjugated polymers. Over the past several decades, a wide variety of π-conjugated polymers have been synthesized to realize the desired properties and improve the performance of organic electronic devices. However, this strategy is not suitable for generating soluble 2D-π-conjugated materials, including ladder polymers, nanoribbons, and 2D-π-conjugated polymers, due to strong van der Waals interactions between the ribbons and sheets. The drive to synthesize higher dimensional polymers and to enhance polymers' properties has spurred the exploration of a novel direction in materials chemistry—the synthesis of unconventional monomers and polymers. The Gavvalapalli research group has developed and used cycloalkyl straps containing aryl building blocks for the synthesis of conjugated polymers. These cycloalkyl straps, positioned either above or below the π-conjugation plane, have been shown to directly control the π–π interactions between the polymer chains. We have demonstrated that π-face masking cycloalkyl straps hinder interchain π–π interactions. The first part of this review article highlights the use of cycloalkyl straps for the synthesis of higher dimensional π-conjugated polymers. In this section, we discuss the synthesis of 2D-H-mers, dispersible hyperbranched π-conjugated polymers, and conjugated porous polymers without the pendant solubilizing chains. The second part of the feature article highlights how the cycloalkyl straps can be used to gain control over polymer–acceptor interactions, including the interaction strength and the location of the acceptor along the polymer backbone. We conclude the article with the future outlook on cycloalkyl strap-containing building blocks in the world of conjugated polymers.
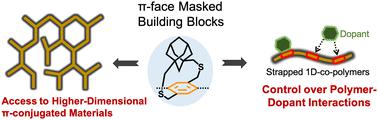
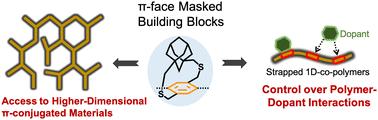
π-共轭聚合物的进展:利用环烷基带开发高性能π-共轭材料。
悬烷基链被广泛用于成功获得各种可溶性线性一维 π 共轭聚合物。在过去的几十年中,人们合成了各种各样的 π 共轭聚合物,以实现所需的性能并提高有机电子器件的性能。然而,这种策略并不适合生成可溶的二维-π-共轭材料,包括梯形聚合物、纳米带和二维-π-共轭聚合物,原因是带和片之间存在很强的范德华相互作用。合成更高维聚合物和增强聚合物性能的动力促使人们探索材料化学的新方向--合成非常规单体和聚合物。Gavvalapalli 研究小组开发并使用了含有芳基结构单元的环烷基带,用于合成共轭聚合物。这些环烷基带位于π-共轭平面的上方或下方,已被证明可以直接控制聚合物链之间的π-π相互作用。我们已经证明,π 面掩蔽环烷基带阻碍了链间 π-π 的相互作用。本综述文章的第一部分重点介绍了利用环烷基链合成高维 π 共轭聚合物的方法。在这一部分中,我们讨论了二维-H-聚合物、可分散超支化π-共轭聚合物以及无悬垂增溶链的共轭多孔聚合物的合成。专题文章的第二部分重点介绍了如何利用环烷基带控制聚合物与受体之间的相互作用,包括相互作用强度和受体在聚合物骨架上的位置。文章最后展望了含环烷基带构筑模块在共轭聚合物领域的未来前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


