Exercise training attenuates cardiac dysfunction induced by excessive sympathetic activation through an AMPK-KLF4-FMO2 axis
IF 4.9
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Cardiovascular diseases (CVDs) are a leading cause of mortality worldwide and are associated with an overactivated sympathetic system. Although exercise training has shown promise in mitigating sympathetic stress-induced cardiac remodeling, the precise mechanisms remain elusive. Here, we demonstrate that exercise significantly upregulates cardiac flavin-containing monooxygenase 2 (FMO2) expression. Notably, we find that exercise training effectively counteracts sympathetic overactivation-induced cardiac dysfunction and fibrosis by enhancing FMO2 expression via adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) activation. Functional investigations employing FMO2 knockdown with adeno-associated virus 9 (AAV9) underscore the necessity for FMO2 expression to protect the heart during exercise in vivo. Furthermore, we identify the krüppel-like factor 4 (KLF4) as a transcriptional mediator of FMO2 that is crucial for the mechanism through which AMPK activation protects against sympathetic overactivation-induced cardiac dysfunction and fibrosis. Taken together, our study reveals a cardioprotective mechanism for exercise training through an AMPK-KLF4-FMO2 signaling pathway that underscores how exercise alleviates cardiac dysfunction induced by excessive sympathetic activation.
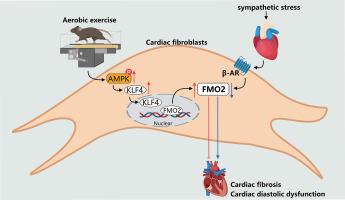
通过AMPK-KLF4-FMO2轴,运动训练可减轻交感神经过度激活引起的心脏功能障碍。
心血管疾病(CVD)是导致全球死亡的主要原因,与交感神经系统过度激活有关。虽然运动训练有望减轻交感神经压力诱导的心脏重塑,但其确切机制仍难以捉摸。在这里,我们证明了运动能显著上调心脏含黄素单加氧酶 2(FMO2)的表达。值得注意的是,我们发现运动训练可通过激活腺苷-5'-单磷酸(AMP)激活蛋白激酶(AMPK)来提高FMO2的表达,从而有效抵消交感神经过度激活诱导的心功能障碍和纤维化。利用腺相关病毒9(AAV9)敲除FMO2的功能研究强调了FMO2的表达对于在体内运动时保护心脏的必要性。此外,我们还发现克鲁佩尔样因子 4(KLF4)是 FMO2 的转录介质,对于 AMPK 激活保护交感神经过度激活诱发的心脏功能障碍和纤维化的机制至关重要。综上所述,我们的研究揭示了运动训练通过 AMPK-KLF4-FMO2 信号通路保护心脏的机制,强调了运动如何缓解交感神经过度激活诱导的心脏功能障碍。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
0.00%
发文量
171
审稿时长
42 days
期刊介绍:
The Journal of Molecular and Cellular Cardiology publishes work advancing knowledge of the mechanisms responsible for both normal and diseased cardiovascular function. To this end papers are published in all relevant areas. These include (but are not limited to): structural biology; genetics; proteomics; morphology; stem cells; molecular biology; metabolism; biophysics; bioengineering; computational modeling and systems analysis; electrophysiology; pharmacology and physiology. Papers are encouraged with both basic and translational approaches. The journal is directed not only to basic scientists but also to clinical cardiologists who wish to follow the rapidly advancing frontiers of basic knowledge of the heart and circulation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


