Lithium iodide promoted CO2 hydrogenation towards ethanol via biphasic lewis-acid-base pairs synergistic catalysis
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
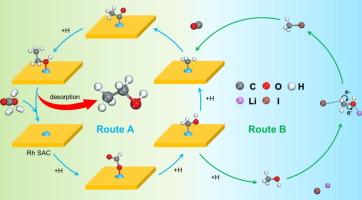
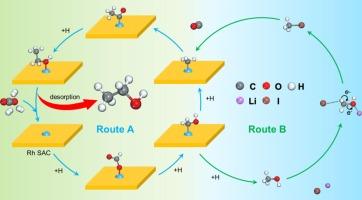
Ethanol synthesis via CO2 hydrogenation is of great importance in view of its high-value utilization. Herein, combining with heterogeneous and homogenous catalysis, we have developed an efficient biphasic Lewis-acid-base pairs synergistic catalysis strategy from CO2 hydrogenation. Apart from conventional heterogeneous ethanol synthesis route, it was surprisingly found that LiI homogenous promoter significantly enhanced CO2 conversion, ethanol selectivity, and productivity. LiI promoted CO2 dissolution in pure water and accelerated reaction intermediates consumption, favoring CO2 conversion. In detail, it can weaken C-O bond of CH3OH intermediate through Li+-I- Lewis-acid-base catalysis. The formed CH3I* with lower bond energy for C-I was more facile to dissociate into CH3*, favoring its subsequent coupling with CO* to form ethanol. In combination of Rh1/CeTiOx catalyst, LiI promoter, and methanol as solvent, a record-breaking ethanol yield was achieved (223.1 mmol·g−1·h−1). The obtained ethanol yield was about 18.7 times of that reported the best result in literatures. Especially, the established catalyst system could also be applied for synthesis of higher-carbon-number alcohols by the CO2 hydrogenation with other alcohols solvent (CnH2n+1OH, n = 2,3…).
碘化锂通过双相路易斯酸碱对协同催化促进二氧化碳加氢制乙醇
二氧化碳加氢合成乙醇具有重要的高值化利用价值。在此,我们结合异相催化和均相催化,开发了一种高效的二氧化碳加氢双相路易斯酸碱对协同催化策略。除了传统的异相乙醇合成路线外,我们还惊喜地发现 LiI 均相促进剂能显著提高二氧化碳转化率、乙醇选择性和生产率。LiI 能促进二氧化碳在纯水中的溶解,加速反应中间产物的消耗,有利于二氧化碳的转化。具体而言,它能通过 Li+-I- 路易斯酸碱催化作用削弱 CH3OH 中间体的 C-O 键。形成的 CH3I* 与 C-I 的键能较低,更容易解离成 CH3*,有利于其随后与 CO* 偶联生成乙醇。结合使用 Rh1/CeTiOx 催化剂、LiI 促进剂和甲醇作为溶剂,乙醇产量达到了破纪录的水平(223.1 mmol-g-1-h-1)。所获得的乙醇产量约为文献报道最佳结果的 18.7 倍。特别是,所建立的催化剂体系还可应用于以其他醇类溶剂(CnH2n+1OH,n = 2、3......)进行 CO2 加氢合成更高碳数的醇类。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


