Harnessing Electrolyte Chemistry to Advance Oxygen Reduction Catalysis for Fuel Cells and Electrosynthesis
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
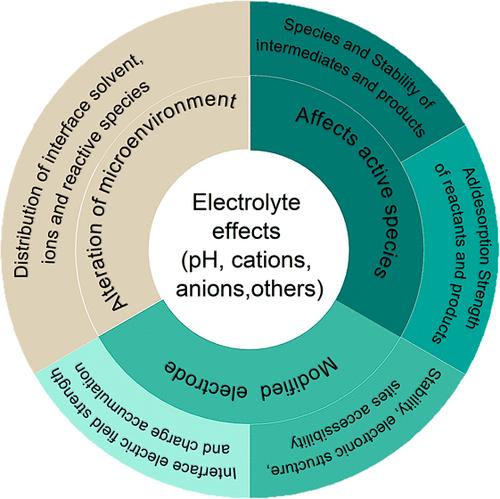
Oxygen reduction reaction (ORR) is ubiquitous in many important energy conversion technologies, encompassing fuel cells, metal-air batteries, and H2O2 electrosynthesis. However, its inherently sluggish kinetics often leads to substantial overpotentials and losses in efficiency, thus prompting extensive efforts into catalyst optimization. In the past few years, growing research has underscored the pivotal role of electrolyte-associated factors in affecting the ORR performance. In this review, we focus on the intricate interplay between electrolyte properties, pH, cations, anions, and additives, and their impacts on ORR electrocatalysis, particularly for platinum (and its alloys) and nonprecious metal–nitrogen–carbon catalysts. We examine how these electrolyte-mediated alterations affect the electrode surface, reactive species, and microenvironment, thereby modulating the adsorption energetics of intermediates, catalyst stability and mass transport, and ultimately affecting the overall ORR process. We highlight the need for dynamic models and advanced probing technologies at the electrocatalytic interfaces, and advocate for adopting a holistic approach that synchronizes effects of electrolytes and catalysts in optimizing ORR electrocatalysis. This review lays the foundation for refining descriptive formulations for ORR electrocatalysis, which potentially guides the development of enhanced ORR cathodes for practical applications.
利用电解质化学推进燃料电池和电合成中的氧还原催化反应
氧还原反应(ORR)在许多重要的能源转换技术中无处不在,包括燃料电池、金属空气电池和 H2O2 电合成。然而,其固有的缓慢动力学往往会导致大量的过电位和效率损失,从而促使人们在催化剂优化方面做出大量努力。在过去几年中,越来越多的研究强调了电解质相关因素在影响 ORR 性能中的关键作用。在本综述中,我们将重点讨论电解质特性、pH 值、阳离子、阴离子和添加剂之间错综复杂的相互作用及其对 ORR 电催化的影响,尤其是对铂(及其合金)和非贵金属氮碳催化剂的影响。我们研究了这些电解质介导的变化如何影响电极表面、反应物和微环境,从而调节中间产物的吸附能、催化剂稳定性和质量传输,并最终影响整个 ORR 过程。我们强调了在电催化界面建立动态模型和采用先进探测技术的必要性,并主张采用一种整体方法,在优化 ORR 电催化过程中同步考虑电解质和催化剂的影响。这篇综述为完善 ORR 电催化的描述性公式奠定了基础,从而有可能指导开发用于实际应用的增强型 ORR 阴极。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


