Delivery of imiquimod to intestinal lymph nodes following oral administration
IF 5.2
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Intestinal lymph nodes are involved in the progression of colorectal cancer (CRC). Tumours suppress the activation of dendritic cells (DCs) in draining lymph nodes, diminishing anti-cancer immune response. Imiquimod (IMQ) facilitates DCs activation via toll-like receptor 7, suggesting that targeted delivery of IMQ to intestinal lymph nodes can improve the treatment of CRC. This study aims to enhance the delivery of IMQ to intestinal lymph nodes by a highly lipophilic prodrug approach. Amide prodrugs were synthesised by conjugating IMQ with saturated and unsaturated medium- to long-chain fatty acids. Their potential for intestinal lymphatic transport was assessed by their affinity to chylomicrons and solubility in long-chain triglycerides. Further selection of prodrug candidates was determined by resistance to enzymatic hydrolysis in intestinal lumen and release of IMQ in the lymphatics using fasting state simulated intestinal fluid supplemented with esterases, brush border enzyme vesicles and plasma. Key pharmacokinetic parameters and biodistribution in rats were assessed for the most promising compounds, prodrugs 5 and 8. The plasma concentration–time profile of IMQ following oral administration of the prodrugs was less erratic in comparison to the administration of unmodified IMQ. The lymph-to-plasma ratios of IMQ concentration increased 1.9- and 1.7-fold using prodrugs 5 and 8 in comparison to administration of unmodified IMQ, respectively. Importantly, the average concentration of IMQ in mesenteric lymph nodes (MLN) was 11.2- and 7.6-fold higher than in plasma following the administration of prodrugs 5 and 8, respectively. Additionally, the non-specific wide distribution of IMQ into various organs and tissues was reduced with prodrugs. This work suggests that the highly lipophilic prodrug approach can efficiently deliver IMQ to intestinal lymphatics. In addition, this study demonstrates the feasibility of an amide prodrug approach for intestinal lymphatic targeting.
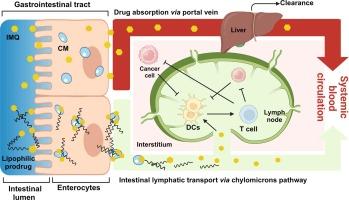
口服咪喹莫特后向肠道淋巴结输送。
肠道淋巴结与结直肠癌(CRC)的进展有关。肿瘤会抑制引流淋巴结中树突状细胞(DC)的活化,从而削弱抗癌免疫反应。咪喹莫特(IMQ)可通过收费样受体7促进树突状细胞的活化,这表明向肠淋巴结靶向递送IMQ可改善对CRC的治疗。本研究旨在通过一种高亲脂性原药的方法,加强IMQ向肠淋巴结的递送。通过将 IMQ 与饱和及不饱和中长链脂肪酸共轭,合成了酰胺原药。这些原药与乳糜微粒的亲和力以及在长链甘油三酯中的溶解度评估了它们在肠道淋巴运输方面的潜力。利用空腹状态下的模拟肠液,并辅以酯酶、刷状边界酶囊和血浆,通过对肠腔内酶水解的耐受性和 IMQ 在淋巴管中的释放情况,进一步筛选出候选原药。对最有前景的化合物、原药 5 和 8 的主要药代动力学参数和在大鼠体内的生物分布进行了评估。与口服未改性的 IMQ 相比,口服原药后 IMQ 的血浆浓度-时间曲线波动较小。与服用未改性的 IMQ 相比,使用原药 5 和 8 后 IMQ 的淋巴-血浆浓度比分别增加了 1.9 倍和 1.7 倍。重要的是,使用原药 5 和 8 后,肠系膜淋巴结(MLN)中的 IMQ 平均浓度分别是血浆中的 11.2 倍和 7.6 倍。此外,原药还减少了 IMQ 在各器官和组织中的非特异性广泛分布。这项工作表明,高亲脂性原药方法可以有效地将 IMQ 送入肠道淋巴管。此外,这项研究还证明了酰胺原药方法用于肠道淋巴靶向的可行性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


