Transitioning from Pickering emulsions to Pickering emulsion hydrogels: A potential advancement in cosmeceuticals
IF 4.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2024-10-30
DOI:10.1016/j.ejpb.2024.114572
引用次数: 0
Abstract
Cosmeceuticals, focusing on enhancing skin health and appearance, heavily rely on emulsions as one of the common mediums. These emulsions pose a challenge due to their dependence on surfactants which are essential for stability but are causing concerns about environmental impact as well as evolving consumer preferences. This has led to research focused on Pickering emulsions (PEs), which are colloidal particle-based emulsion alternatives. Compared to conventional emulsions, PEs offer enhanced stability and functionality in addition to serving as a sustainable alternative but still pose challenges such as rheological control and requiring further improvement in long-term stability, whereby the limitations could be addressed through the introduction of a hydrogel network. In this review, we first highlight the strategies and considerations to optimize active ingredient (AI) absorption and penetration in a PE-based formulation. We then delve into a comprehensive overview of the potential of Pickering-based cosmeceutical emulsions including their attractive features, the various Pickering particles that can be employed, past studies and their limitations. Further, PE hydrogels (PEHs), which combines the features between PE and hydrogel as an innovative solution to address challenges posed by both conventional emulsions and PEs in the cosmeceutical industry is explored. Moreover, concerns related to toxicity and biocompatibility are critically examined, alongside considerations of scalability and commercial viability, providing a forward-looking perspective on potential future research directions centered on the application of PEHs in the cosmeceutical field.
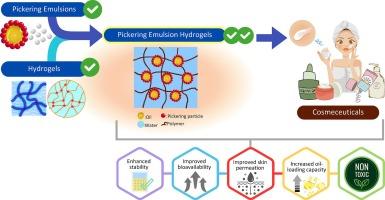
从皮克林乳液过渡到皮克林乳液水凝胶:药用化妆品的潜在进步。
注重增强皮肤健康和外观的药妆产品在很大程度上依赖乳液作为常用介质之一。这些乳液对表面活性剂的依赖是一个挑战,表面活性剂对稳定性至关重要,但却引起了人们对环境影响和消费者偏好不断变化的担忧。因此,研究重点转向皮克林乳液(PE),这是一种基于胶体颗粒的乳液替代品。与传统乳液相比,皮克林乳液除了作为一种可持续的替代品外,还具有更高的稳定性和功能性,但仍面临流变控制等挑战,需要进一步提高长期稳定性,而这些限制可通过引入水凝胶网络来解决。在本综述中,我们首先强调了在聚乙烯制剂中优化活性成分(AI)吸收和渗透的策略和注意事项。然后,我们全面概述了基于皮克林的药用化妆品乳剂的潜力,包括其诱人的特点、可采用的各种皮克林颗粒、以往的研究及其局限性。此外,还探讨了聚乙烯水凝胶(PEHs),它结合了聚乙烯和水凝胶的特点,是解决传统乳剂和聚乙烯在药用化妆品行业所面临挑战的创新解决方案。此外,还批判性地研究了与毒性和生物相容性有关的问题,以及可扩展性和商业可行性方面的考虑因素,为以聚乙烯水凝胶在药妆领域的应用为中心的潜在未来研究方向提供了前瞻性视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


