Overexpression of MCL1 attenuates irritable bowel syndrome by regulating cuproptosis: Screening and validation
IF 2.5
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochemical and biophysical research communications
Pub Date : 2024-10-29
DOI:10.1016/j.bbrc.2024.150926
引用次数: 0
Abstract
Irritable bowel syndrome (IBS) is a type of chronic bowel disorder with a poorly understood pathophysiology. Recently, the imbalance of copper has been reported to influence the progression of IBS, suggesting cuproptosis, a new type of copper-induced cell death, may play a role in IBS. This study found 17 cuproptosis-related differentially expressed genes in IBS through bioinformatic analysis. Six hub genes were identified after the protein-protein interaction network analysis, namely myeloid cell leukemia 1 (MCL1), epidermal growth factor receptor 2, cadherin-associated protein beta 1, solute carrier family 25 members 37, solute carrier family 39 members 14, and six transmembrane epithelial antigens of the prostate 3. We selected MCL1 for further verification. Human normal colon epithelial cell line (NCM460) was used to construct models of IBS or cuproptosis in vitro by lipopolysaccharide (LPS) or LPS combined with copper (II) chloride (CuCl2). We observed that overexpression of MCL1 promoted cell viability and proliferation ability, and inhibited the secretion of inflammatory factors and expression of Bax and caspase-3 of NCM460 cells treated with LPS or LPS combined with CuCl2. In addition, up-regulated MCL1 significantly suppressed the protein levels of ferredoxin 1 and lipoyl synthase, two key regulators of cuproptosis. In conclusion, our study demonstrates that cuproptosis is involved in IBS and identifies a cuproptosis-related gene, MCL1, that helps alleviate IBS by promoting cell growth, reducing inflammation, and suppressing cuproptosis, making it a promising therapeutic target in IBS.
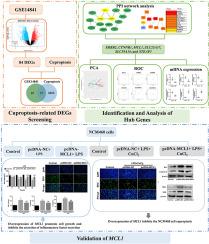
过表达 MCL1 可通过调节杯突减少肠易激综合征:筛选与验证
肠易激综合征(IBS)是一种病理生理学尚不清楚的慢性肠道疾病。最近,有报道称铜的失衡会影响肠易激综合征的进展,这表明铜诱导的一种新型细胞死亡--杯突症可能在肠易激综合征中发挥作用。本研究通过生物信息学分析发现了 17 个与杯突症相关的 IBS 差异表达基因。经过蛋白质-蛋白质相互作用网络分析,我们发现了六个中心基因,分别是髓细胞白血病 1(MCL1)、表皮生长因子受体 2、粘连蛋白相关蛋白 beta 1、溶质运载家族 25 成员 37、溶质运载家族 39 成员 14 和前列腺 6 个跨膜上皮抗原 3。我们选择了 MCL1 进行进一步验证。我们利用人体正常结肠上皮细胞系(NCM460)通过脂多糖(LPS)或 LPS 结合氯化铜(CuCl2)在体外构建肠易激综合征或杯突症模型。我们观察到,过表达 MCL1 能提高细胞活力和增殖能力,抑制 LPS 或 LPS 结合 CuCl2 处理的 NCM460 细胞的炎症因子分泌、Bax 和 Caspase-3 的表达。此外,上调的 MCL1 能显著抑制铁氧还蛋白 1 和脂酰合酶(杯突变的两个关键调节因子)的蛋白水平。总之,我们的研究证明了杯突症与肠易激综合征有关,并发现了一个与杯突症相关的基因--MCL1,它通过促进细胞生长、减轻炎症和抑制杯突症来帮助缓解肠易激综合征,使其成为肠易激综合征的一个有希望的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.10
自引率
0.00%
发文量
1400
审稿时长
14 days
期刊介绍:
Biochemical and Biophysical Research Communications is the premier international journal devoted to the very rapid dissemination of timely and significant experimental results in diverse fields of biological research. The development of the "Breakthroughs and Views" section brings the minireview format to the journal, and issues often contain collections of special interest manuscripts. BBRC is published weekly (52 issues/year).Research Areas now include: Biochemistry; biophysics; cell biology; developmental biology; immunology
; molecular biology; neurobiology; plant biology and proteomics

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


