Copper Oxide Nanoparticles Impair Mouse Preimplantation Embryonic Development through Disruption of Mitophagy-Mediated Metabolism
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Copper oxide nanoparticles (CuONPs) have been widely applied, posing potential risks to human health. Although the toxicity of CuONPs on the liver and spleen has been reported, their effects on reproductive health remain unexplored. In this study, we investigate the effects of CuONPs on embryonic development and their potential mechanisms. Our results demonstrate that CuONPs exposure impairs mouse preimplantation embryonic development, particularly affecting the morula-to-blastocyst transition. Additionally, CuONPs were found to reduce the pluripotency of the inner cell mass (ICM) and mouse embryonic stem cells (mESCs). Mechanistically, CuONPs block autophagic flux and impair mitophagy, leading to the accumulation of damaged mitochondria. This mitochondrial dysfunction leads to reduced tricarboxylic acid (TCA) cycle activity and decreased α-ketoglutarate (α-KG) production. Insufficient α-KG induces the failure of DNA demethylation, reducing corresponding chromatin accessibility and consequently inhibiting ICM-specific genes expressions. Similar reduced development and inhibitions of pluripotency gene expression were observed in CuONPs-treated human blastocysts. Moreover, in women undergoing assisted reproductive technology (ART), a negative correlation was found between urinary Cu ion concentrations and clinical outcomes. Collectively, our study elucidates the mitophagy-mediated metabolic mechanisms of CuONPs embryotoxicity, improving our understanding of the potential reproductive toxicity associated with it.
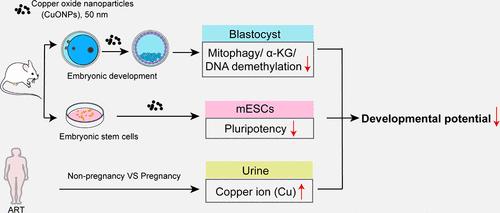
纳米氧化铜颗粒通过破坏有丝分裂介导的新陈代谢损害小鼠植入前胚胎发育
纳米氧化铜(CuONPs)已被广泛应用,对人类健康构成潜在风险。虽然 CuONPs 对肝脏和脾脏的毒性已有报道,但其对生殖健康的影响仍有待探索。本研究调查了 CuONPs 对胚胎发育的影响及其潜在机制。我们的研究结果表明,接触 CuONPs 会损害小鼠胚胎植入前的发育,尤其是影响卵泡到囊胚的转变。此外,我们还发现 CuONPs 会降低内细胞团(ICM)和小鼠胚胎干细胞(mESCs)的多能性。从机理上讲,CuONPs 会阻碍自噬通量并损害有丝分裂,从而导致受损线粒体的积累。这种线粒体功能障碍会导致三羧酸(TCA)循环活性降低和α-酮戊二酸(α-KG)生成减少。α-KG不足会导致DNA去甲基化失败,降低相应的染色质可及性,从而抑制ICM特异性基因的表达。在经 CuONPs 处理的人类胚泡中也观察到了类似的发育减弱和多能基因表达抑制现象。此外,在接受辅助生殖技术(ART)的妇女中,发现尿液中铜离子浓度与临床结果呈负相关。总之,我们的研究阐明了 CuONPs 胚胎毒性由有丝分裂介导的代谢机制,提高了我们对其潜在生殖毒性的认识。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


