Genetic associations of metabolic factors and therapeutic drug targets with polycystic ovary syndrome
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
Polycystic ovary syndrome (PCOS) is frequently accompanied with metabolic dysfunctions, yet the causal relationships between metabolic factors and PCOS remain to be conclusively established and etiology-based therapies are lacking.Objectives
To comprehensively identify the metabolic causal factors and potential drug targets for PCOS.Methods
This genetic association study was conducted using bidirectional two-sample Mendelian Randomization (MR), multivariable MR (MVMR) and drug-target MR. Considering metabolic sexual dimorphism, female-specific genome-wide association studies (GWASs) for metabolic factors were obtained. To ensure the robustness of the findings, an additional independent PCOS GWAS dataset was utilized for replication.Results
The PCOS cohort included 10,074 PCOS cases (mean age 28 to 45 years) and 103,164 controls (mean age 27 to 60 years) of European ancestry. All participants were female. Employing two-sample MR analysis, we found that genetically proxied body mass index (BMI) (OR = 3.40 [95 % CI, 2.65–4.36]), triglyceride (TG) (OR = 1.54 [95 % CI, 1.17–2.04]), low-density lipoprotein cholesterol (LDL-c) (OR = 1.37 [95 % CI, 1.07–1.76]), and type 2 diabetes (T2D) (OR = 1.24 [95 % CI, 1.09–1.41]) were significantly associated with an increased risk of PCOS, whereas genetically predicted high-density lipoprotein cholesterol (HDL-c) (OR = 0.61 [95 % CI, 0.47–0.80]) decreased the odds of PCOS. Stepwise MVMR established a hierarchy of interactions among these metabolic factors, identifying BMI and HDL-c as the most prominent causal factors. Notably, drug-target MR analysis identified incretin-based therapeutics, PCSK9 inhibitors, LPL gene therapy, sulfonylureas, and thiazolidinediones as potential therapeutics for PCOS. All these findings were validated in an independent dataset.Conclusion
This study offered insights into the roles of obesity, diabetes, and dyslipidemia in PCOS etiology and therapeutics, underscoring the necessity for managing metabolic health in women and paving the way for tailored therapeutic strategies for PCOS based on its metabolic underpinnings.
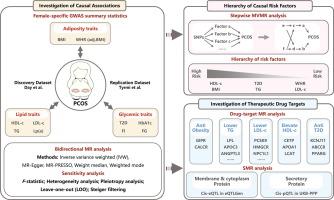
多囊卵巢综合征代谢因素和治疗药物靶点的遗传关联
方法 本遗传关联研究采用双向双样本孟德尔随机化(MR)、多变量 MR(MVMR)和药物靶标 MR 进行。考虑到代谢性双态性,研究人员获得了女性代谢因素的全基因组关联研究(GWAS)。结果多囊卵巢综合征队列包括 10,074 例多囊卵巢综合征病例(平均年龄为 28 至 45 岁)和 103,164 例欧洲血统对照组(平均年龄为 27 至 60 岁)。所有参与者均为女性。通过双样本 MR 分析,我们发现基因代偿的体重指数(BMI)(OR = 3.40 [95 % CI, 2.65-4.36])、甘油三酯(TG)(OR = 1.54 [95 % CI, 1.17-2.04])、低密度脂蛋白胆固醇(LDL-c)(OR = 1.37 [95 % CI, 1.07-1.76])和 2 型糖尿病(T2D)(OR = 1.24 [95 % CI, 1.09-1.41])与多囊卵巢综合征的风险增加显著相关,而遗传预测的高密度脂蛋白胆固醇(HDL-c)(OR = 0.61 [95 % CI, 0.47-0.80])会降低多囊卵巢综合征的几率。逐步MVMR确定了这些代谢因素之间相互作用的层次,确定BMI和HDL-c是最主要的致病因素。值得注意的是,药物靶点 MR 分析确定了增量素类疗法、PCSK9 抑制剂、LPL 基因疗法、磺脲类药物和噻唑烷二酮类药物是治疗多囊卵巢综合症的潜在疗法。结论这项研究深入揭示了肥胖、糖尿病和血脂异常在多囊卵巢综合征病因学和治疗学中的作用,强调了管理女性代谢健康的必要性,并为根据多囊卵巢综合征的代谢基础制定量身定制的治疗策略铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


