Discovery of the first selective and potent PROTAC degrader for the pseudokinase TRIB2
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Pseudokinase TRIB2, a member of the CAMK Ser/Thr protein kinase family, regulates various cellular processes through phosphorylation-independent mechanisms. Dysregulation of TRIB2 has been implicated in promoting tumor growth, metastasis, and therapy resistance, making it a promising target for cancer treatment. In this study, we designed and synthesized a series of TRIB2 PROTAC degraders by conjugating a TRIB2 binder 1 with VHL or CRBN ligands via linkers of varying lengths and compositions. Among these compounds, 5k demonstrated potent TRIB2 degradation with a DC50 value of 16.84 nM (95 % CI: 13.66–20.64 nM) in prostate cancer PC3 cells. Mechanistic studies revealed that 5k directly interacted with TRIB2, selectively inducing its degradation through a CRBN-dependent ubiquitin-proteasomal pathway. Moreover, 5k outperformed the TRIB2 binder alone in inhibiting cell proliferation and inducing apoptosis, confirming that TRIB2 protein degradation could be a promising therapeutic strategy for TRIB2-associated cancers. Additionally, compound 5k also serves as an effective tool for probing TRIB2 biology.
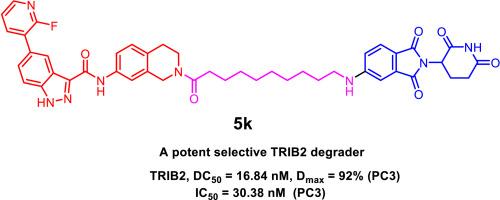
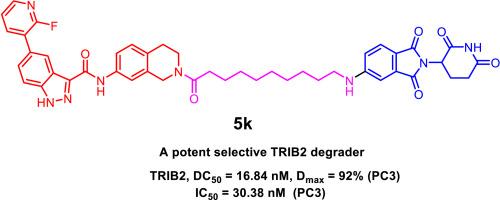
发现首个针对伪激酶 TRIB2 的选择性强效 PROTAC 降解剂
伪激酶 TRIB2 是 CAMK Ser/Thr 蛋白激酶家族的成员,通过磷酸化无关机制调节各种细胞过程。TRIB2 的失调与促进肿瘤生长、转移和耐药性有关,因此是一个很有前景的癌症治疗靶点。在这项研究中,我们设计并合成了一系列 TRIB2 PROTAC 降解剂,方法是通过不同长度和组成的连接体将 TRIB2 结合剂 1 与 VHL 或 CRBN 配体连接。在这些化合物中,5k 对前列腺癌 PC3 细胞的 TRIB2 降解效果显著,DC50 值为 16.84 nM(95% CI:13.66 - 20.64 nM)。机理研究显示,5k 与 TRIB2 直接相互作用,通过依赖 CRBN 的泛素-蛋白酶体途径选择性地诱导其降解。此外,5k 在抑制细胞增殖和诱导细胞凋亡方面的表现优于单独的 TRIB2 结合剂,这证实了 TRIB2 蛋白降解可能是治疗 TRIB2 相关癌症的一种有前景的策略。此外,化合物 5k 还是探究 TRIB2 生物学特性的有效工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


