Spatiotemporal coordination of actin regulators generates invasive protrusions in cell–cell fusion
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
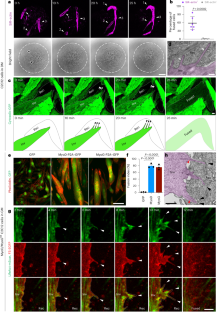
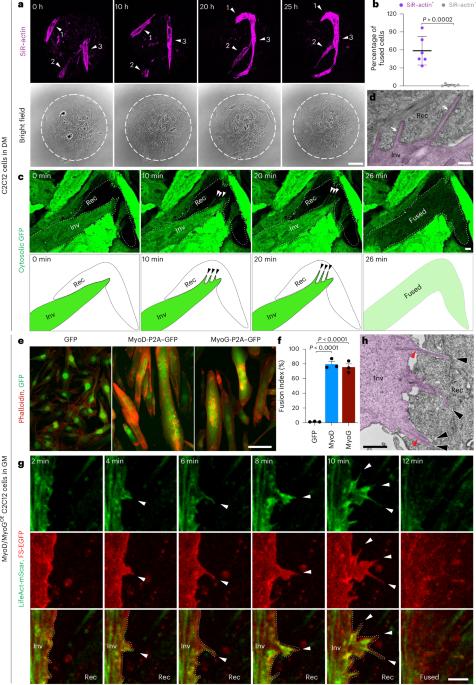
Invasive membrane protrusions play a central role in a variety of cellular processes. Unlike filopodia, invasive protrusions are mechanically stiff and propelled by branched actin polymerization. However, how branched actin filaments are organized to create finger-like invasive protrusions is unclear. Here, by examining the mammalian fusogenic synapse, where invasive protrusions are generated to promote cell membrane juxtaposition and fusion, we have uncovered the mechanism underlying invasive protrusion formation. We show that two nucleation-promoting factors for the Arp2/3 complex, WAVE and N-WASP, exhibit different localization patterns in the protrusions. Whereas WAVE is closely associated with the plasma membrane at the leading edge of the protrusive structures, N-WASP is enriched with WIP along the actin bundles in the shafts of the protrusions. During protrusion initiation and growth, the Arp2/3 complex nucleates branched actin filaments to generate low-density actin clouds in which the large GTPase dynamin organizes the new branched actin filaments into bundles, followed by actin-bundle stabilization by WIP, the latter functioning as an actin-bundling protein. Disruption of any of these components results in defective protrusions and failed myoblast fusion in cultured cells and mouse embryos. Together, our study has revealed the intricate spatiotemporal coordination between two nucleation-promoting factors and two actin-bundling proteins in building invasive protrusions at the mammalian fusogenic synapse and has general implications in understanding invasive protrusion formation in cellular processes beyond cell–cell fusion. Lu et al. reveal the spatiotemporal coordination between two nucleation-promoting factors, WAVE and N-WASP, and two actin-bundling proteins, dynamin and WIP, in generating invasive protrusions at the mammalian myoblast fusogenic synapse.
肌动蛋白调节因子的时空协调在细胞-细胞融合中产生侵袭性突起
侵袭性膜突起在多种细胞过程中发挥着核心作用。与丝状突起不同,侵袭性突起具有机械硬度,并由分枝肌动蛋白聚合推动。然而,分枝肌动蛋白丝是如何组织起来形成指状侵入性突起的还不清楚。在这里,我们通过研究哺乳动物的融合突触(在这种突触中,侵入性突起的产生促进了细胞膜的并置和融合),揭示了侵入性突起形成的机制。我们发现 Arp2/3 复合物的两个成核促进因子 WAVE 和 N-WASP 在突起中表现出不同的定位模式。WAVE 与突起结构前缘的质膜密切相关,而 N-WASP 则与 WIP 一起沿突起轴的肌动蛋白束富集。在突起萌发和生长过程中,Arp2/3 复合物核化分枝肌动蛋白丝,生成低密度肌动蛋白云,其中大 GTP 酶达能蛋白将新的分枝肌动蛋白丝组织成束,然后由 WIP 稳定肌动蛋白束,后者具有肌动蛋白束蛋白的功能。在培养细胞和小鼠胚胎中,这些成分中任何一个被破坏,都会导致突起缺陷和成肌细胞融合失败。总之,我们的研究揭示了两种成核促进因子和两种肌动蛋白束缚蛋白在哺乳动物融合突触形成侵袭性突起过程中错综复杂的时空协调,对理解细胞-细胞融合以外的细胞过程中侵袭性突起的形成具有普遍意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


