1,3-Butadiynyl sulfide-based compact trialkyne platform molecule for sequential assembly of three azides†
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A compact trialkyne platform with a silyl-protected 1,3-butadiynyl sulfide moiety and a terminal alkyne group has been developed for sequential regioselective transition metal-catalyzed triazole formation reactions with three azides. This method enabled the facile construction of a low-molecular-weight triazole library and the synthesis of middle-molecular-weight trifunctional probes for protein modification.
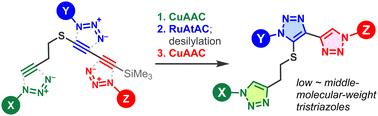
基于 1,3-丁二炔硫醚的紧凑型三炔基平台分子,用于三种叠氮化物的顺序组装
我们开发了一个紧凑的三炔基平台,该平台具有硅基保护的 1,3-丁二炔硫醚分子和一个末端炔基,可用于过渡金属催化的三唑与三种叠氮化物的连续区域选择性生成反应。通过这种方法,可以方便地构建低分子量的三唑库,并合成用于蛋白质修饰的中等分子量的三官能探针。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


