Unveiling the Pivotal Role of Ce Coordination Structures and Their Surface Arrangements in Governing 2-Cyanopyridine Hydrolysis for Direct Dimethyl Carbonate Synthesis from CO2 and Methanol
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
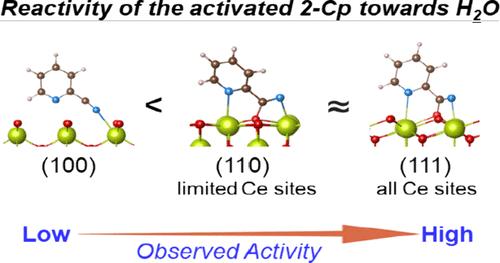
The direct synthesis of dimethyl carbonate (DMC) from CO2 and methanol presents a promising alternative to conventional methods that use toxic chemicals, but its yield is limited by equilibrium. Coupling this reaction with 2-cyanopyridine (2-Cp) hydrolysis over CeO2-based catalysts was found to significantly boost the DMC yield by removing water. Our recent study has revealed that methanol is the key species being activated by surface Ce sites to produce DMC. The reactivity of surface methoxy species toward CO2 varies greatly with their configuration, which is determined by the Ce coordination structures. A similar challenge remains in understanding the CeO2 surface feature governing the hydrolysis of 2-Cp to 2-picolinamide (2-PA). Herein, CeO2 nanocrystallites with well-defined (111), (110), and (100) surfaces were used to study the effects of Ce coordination structures and their arrangements in this reaction and coupled DMC synthesis. We found that the synergistic adsorption of 2-Cp via cyano-N and pyridine-N on (111) and (110) surfaces enables nucleophilic addition of lattice oxygen, producing imino-like N with stronger Lewis basicity, which in turn facilitates hydrolysis. The (111) surface outperforms the (110) surface due to its unique Ce coordination structure and arrangement, which allows more 2-Cp activation and easier 2-PA desorption. Notably, the (111)-enclosed octahedral CeO2 used herein outperforms the reported pristine CeO2 catalysts in this coupled reaction. In contrast, this synergistic adsorption/activation does not occur on the (100) surface, leading to low activity. These findings provide insights for designing CeO2-based catalysts for CO2 conversion with alcohols and amines using 2-Cp as a dehydrant.
揭示 Ce 配位结构及其表面排列在调控 2-氰基吡啶水解以二氧化碳和甲醇直接合成碳酸二甲酯过程中的关键作用
与使用有毒化学品的传统方法相比,从二氧化碳和甲醇直接合成碳酸二甲酯(DMC)是一种很有前景的替代方法,但其产量受到平衡的限制。研究发现,将这一反应与 2-氰基吡啶(2-Cp)在 CeO2 基催化剂上的水解作用结合起来,可以通过去除水分而显著提高 DMC 的产率。我们最近的研究发现,甲醇是表面 Ce 位点激活生成 DMC 的关键物种。表面甲氧基物种对 CO2 的反应活性因其构型而有很大不同,这是由 Ce 配位结构决定的。要了解 CeO2 表面如何将 2-Cp 水解为 2-吡啶酰胺 (2-PA),也面临着类似的挑战。在此,我们利用具有明确定义的 (111)、(110) 和 (100) 表面的 CeO2 纳米晶来研究 Ce 配位结构及其排列在该反应和耦合 DMC 合成中的影响。我们发现,2-Cp 通过氰基-N 和吡啶-N 在(111)和(110)表面的协同吸附作用,使晶格氧发生亲核加成,产生具有更强路易斯碱性的亚胺样 N,进而促进水解。(111)表面的性能优于(110)表面,这是因为其独特的Ce配位结构和排列方式允许更多的2-Cp活化和更容易的2-PA解吸。值得注意的是,此处使用的 (111) 封闭八面体 CeO2 在此耦合反应中的性能优于已报道的原始 CeO2 催化剂。相比之下,这种协同吸附/活化作用在 (100) 表面没有发生,导致活性较低。这些发现为设计基于 CeO2 的催化剂提供了启示,以便利用 2-Cp 作为脱水剂将二氧化碳与醇类和胺类进行转化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


