Synergistic structural integrity and remarkable structural stability of NC@Si anodes for lithium-ion batteries
IF 5.3
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
The silicon-based anode has attracted wide attention because of its high theoretical specific capacity, which is expected to greatly improve the batteries’ energy density. However, during the charge-discharge cycles, the great volume expansion of the anode active silicon seriously restricts its electrochemical stability. Herein, N-doping carbon-coated silicon microsphere was prepared for lithium-ion batteries. Benefit from the core-shell structure formed from silicon carbon, the volume change of silicon anode during the cycle is well coordinated. What's more, N-doping carbon reduces the reaction energy barrier between silicon and lithium and accelerates the electrochemical reaction, which improves the cycling performance of the battery. The specific capacity of the prepared button battery is as high as 2913.13 mAh g−1 at the current density of 0.21 mA g−1, and the average coulomb efficiency is higher than 99.84 % during the cycling. And the capacity of NC@SiO2 anode gradually stabilizes after 500 cycles.
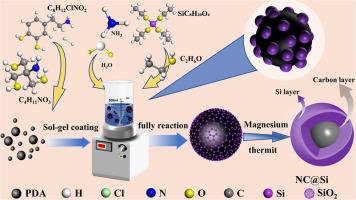
用于锂离子电池的 NC@Si 阳极的协同结构完整性和显著的结构稳定性
硅基负极因其理论比容量高,有望大大提高电池的能量密度而受到广泛关注。然而,在充放电循环过程中,阳极活性硅的巨大体积膨胀严重制约了其电化学稳定性。本文制备了用于锂离子电池的 N 掺杂碳包覆硅微球。得益于硅碳形成的核壳结构,硅负极在循环过程中的体积变化得到了很好的协调。此外,掺杂 N 的碳降低了硅与锂之间的反应能垒,加速了电化学反应,从而提高了电池的循环性能。在 0.21 mA g-1 的电流密度下,制备的纽扣电池的比容量高达 2913.13 mAh g-1,循环过程中的平均库仑效率高于 99.84%。NC@SiO2 阳极的容量在循环 500 次后逐渐趋于稳定。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Research Bulletin
工程技术-材料科学:综合
CiteScore
9.80
自引率
5.60%
发文量
372
审稿时长
42 days
期刊介绍:
Materials Research Bulletin is an international journal reporting high-impact research on processing-structure-property relationships in functional materials and nanomaterials with interesting electronic, magnetic, optical, thermal, mechanical or catalytic properties. Papers purely on thermodynamics or theoretical calculations (e.g., density functional theory) do not fall within the scope of the journal unless they also demonstrate a clear link to physical properties. Topics covered include functional materials (e.g., dielectrics, pyroelectrics, piezoelectrics, ferroelectrics, relaxors, thermoelectrics, etc.); electrochemistry and solid-state ionics (e.g., photovoltaics, batteries, sensors, and fuel cells); nanomaterials, graphene, and nanocomposites; luminescence and photocatalysis; crystal-structure and defect-structure analysis; novel electronics; non-crystalline solids; flexible electronics; protein-material interactions; and polymeric ion-exchange membranes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


