Light harvesting S-scheme g-C3N4/TiO2/M photocatalysts for efficient removal of hazardous moxifloxacin
IF 5.1
3区 材料科学
Q2 MATERIALS SCIENCE, COATINGS & FILMS
引用次数: 0
Abstract
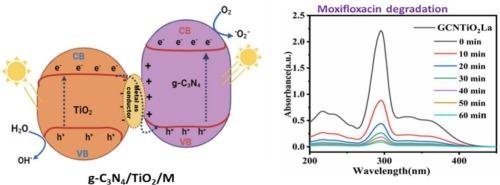
The development of advanced photocatalysts with enhanced efficiency for environmental remediation is critical for addressing persistent organic pollutants like Moxifloxacin. In this study, we present a novel S-scheme heterojunction g-C3N4/TiO2/M photocatalyst designed to optimize light harvesting and charge separation for the effective degradation of Moxifloxacin. The innovative S-scheme configuration enables superior charge transfer dynamics by retaining potent photogenerated electrons in the conduction band of TiO2 and holes in the valence band of g-C3N4, significantly improving photocatalytic performance compared to conventional Type-II systems. Heterogeneous photocatalysis, particularly using TiO2-based materials, offers a promising approach. Here, we synthesized TiO2 hybridized with metal-doped g-C3N4 (GCNTM) via a simple hydrothermal method. The fabricated nanocomposites were characterized using SEM, XRD, FTIR, and UV–Vis (DRS). These GCNTM nanocomposites were employed for the degradation of hazardous Moxifloxacin (MXF) under visible light. Detailed analysis of the photocatalytic mechanism reveals that the synergistic interaction between g-C3N4, TiO2, and the co-catalyst M not only broadens the light absorption spectrum but also enhances the separation and lifespan of charge carriers. Among the synthesized materials, GCNTLa demonstrated the highest degradation efficiency, achieving 96 % removal of MXF. This enhanced activity is attributed to the effective suppression of charge recombination, leading to the generation of reactive species responsible for MXF degradation. Additionally, GCNTM showed remarkable stability and reusability, with only a 6 % reduction in efficiency after five cycles, confirming its high reusability and mechanical stability. Our S-scheme photocatalyst demonstrates a marked increase in the degradation rate of Moxifloxacin under visible light irradiation, highlighting its potential for practical environmental applications.
用于高效去除有害莫西沙星的光收集 S 型 g-C3N4/TiO2/M 光催化剂
开发可提高环境修复效率的先进光催化剂对于解决莫西沙星等持久性有机污染物至关重要。在本研究中,我们提出了一种新型 S 型异质结 g-C3N4/TiO2/M 光催化剂,旨在优化光收集和电荷分离,从而有效降解莫西沙星。创新的 S 型结构可将光生电子保留在 TiO2 的传导带中,将空穴保留在 g-C3N4 的价带中,从而实现卓越的电荷转移动力学,与传统的 II 型系统相比,显著提高了光催化性能。异质光催化,尤其是使用基于 TiO2 的材料,提供了一种前景广阔的方法。在这里,我们通过简单的水热法合成了与掺杂金属的 g-C3N4 (GCNTM)杂化的 TiO2。我们使用 SEM、XRD、FTIR 和 UV-Vis (DRS) 对制备的纳米复合材料进行了表征。这些 GCNTM 纳米复合材料被用于在可见光下降解有害的莫西沙星(MXF)。对光催化机理的详细分析显示,g-C3N4、TiO2 和助催化剂 M 之间的协同作用不仅拓宽了光吸收光谱,还增强了电荷载流子的分离和寿命。在合成的材料中,GCNTLa 的降解效率最高,对 MXF 的去除率达到 96%。这种活性的增强归功于电荷重组的有效抑制,从而产生了导致 MXF 降解的活性物种。此外,GCNTM 还表现出卓越的稳定性和可重复使用性,经过五个循环后,其效率仅降低了 6%,这证明了其高度的可重复使用性和机械稳定性。我们的 S 型光催化剂在可见光照射下明显提高了莫西沙星的降解率,突出了其在实际环境应用中的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Diamond and Related Materials
工程技术-材料科学:综合
CiteScore
6.00
自引率
14.60%
发文量
702
审稿时长
2.1 months
期刊介绍:
DRM is a leading international journal that publishes new fundamental and applied research on all forms of diamond, the integration of diamond with other advanced materials and development of technologies exploiting diamond. The synthesis, characterization and processing of single crystal diamond, polycrystalline films, nanodiamond powders and heterostructures with other advanced materials are encouraged topics for technical and review articles. In addition to diamond, the journal publishes manuscripts on the synthesis, characterization and application of other related materials including diamond-like carbons, carbon nanotubes, graphene, and boron and carbon nitrides. Articles are sought on the chemical functionalization of diamond and related materials as well as their use in electrochemistry, energy storage and conversion, chemical and biological sensing, imaging, thermal management, photonic and quantum applications, electron emission and electronic devices.
The International Conference on Diamond and Carbon Materials has evolved into the largest and most well attended forum in the field of diamond, providing a forum to showcase the latest results in the science and technology of diamond and other carbon materials such as carbon nanotubes, graphene, and diamond-like carbon. Run annually in association with Diamond and Related Materials the conference provides junior and established researchers the opportunity to exchange the latest results ranging from fundamental physical and chemical concepts to applied research focusing on the next generation carbon-based devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


