Oxidation of 4-hydroxybenzoic acid in strongly alkaline and high-salt solutions via ultrasonic-assisted ozone: Helping radioactive waste disposal and environmental safety
IF 8.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
4-Hydroxybenzoic acid (4-HBA), as a gibbsite dissolution inhibitor and typical personal care products (PPCPs), seriously troubles radioactive waste disposal and environmental safety. This work studies the degradation mechanism of 4-HBA in ultrasonic-assisted ozonation in strongly alkaline and high-salt solutions, and comprehensively evaluates its environmental lifetime. Ultrasonic can significantly increase the effect of ozone by 1.52 times, making the degradation rate of 4-HBA reach 63.49 % within 60 min. The better electrochemical performance indicates that the redox reaction between US/O3 system and 4-HBA is more prominent. Experimental analysis and density functional theory (DFT) calculations show that the effects of ![]() OH, 1O2, O2
OH, 1O2, O2![]() −, and others on the degradation of 4-HBA are 56.6 %, 17.8 %, 22.1 %, and 3.5 % respectively, and HO3
−, and others on the degradation of 4-HBA are 56.6 %, 17.8 %, 22.1 %, and 3.5 % respectively, and HO3![]() is the most important precursor for
is the most important precursor for ![]() OH evolution. Mechanistic exploration and DFT calculations show that degradation behavior of 4-HBA in strongly alkaline and high-salt solutions, i.e., decarboxylation reaction, ring opening, hydroxylation, aldol condensation, and hydrogenation, is significantly different from acidic or neutral solutions. Based on the environmental lifetime assessment of the intermediate product, US/O3 technology can help reduce the toxicity of 4-HBA. The US/O3 process is used to remove 4-HBA from strongly alkaline and high-salt solutions, which has huge potential economic benefits in the fields of nuclear waste chemistry and environment.
OH evolution. Mechanistic exploration and DFT calculations show that degradation behavior of 4-HBA in strongly alkaline and high-salt solutions, i.e., decarboxylation reaction, ring opening, hydroxylation, aldol condensation, and hydrogenation, is significantly different from acidic or neutral solutions. Based on the environmental lifetime assessment of the intermediate product, US/O3 technology can help reduce the toxicity of 4-HBA. The US/O3 process is used to remove 4-HBA from strongly alkaline and high-salt solutions, which has huge potential economic benefits in the fields of nuclear waste chemistry and environment.
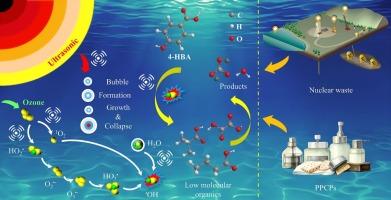
通过超声波辅助臭氧氧化强碱性和高盐溶液中的 4-羟基苯甲酸:有助于放射性废物处理和环境安全
4-羟基苯甲酸(4-HBA)作为一种吉贝岩溶解抑制剂和典型的个人护理产品(PPCPs),严重困扰着放射性废物的处置和环境安全。本文研究了 4-HBA 在强碱性和高盐溶液中超声波辅助臭氧降解的机理,并对其环境寿命进行了综合评价。结果表明,超声波能将臭氧的作用效果明显提高 1.52 倍,使 4-HBA 在 60 分钟内的降解率达到 63.49%。较好的电化学性能表明 US/O3 系统与 4-HBA 之间的氧化还原反应更为突出。实验分析和密度泛函理论(DFT)计算表明,OH、1O2、O2-和其他物质对 4-HBA 降解的影响分别为 56.6%、17.8%、22.1% 和 3.5%,而 HO3 是 OH 演化的最重要前体。机理探索和 DFT 计算表明,4-HBA 在强碱性和高盐溶液中的降解行为,即脱羧反应、开环、羟基化、醛醇缩合和氢化,与酸性或中性溶液有显著不同。根据中间产物的环境寿命评估,US/O3 技术有助于降低 4-HBA 的毒性。US/O3 工艺可用于去除强碱性和高盐溶液中的 4-HBA,在核废料化学和环境领域具有巨大的潜在经济效益。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Desalination
工程技术-工程:化工
CiteScore
14.60
自引率
20.20%
发文量
619
审稿时长
41 days
期刊介绍:
Desalination is a scholarly journal that focuses on the field of desalination materials, processes, and associated technologies. It encompasses a wide range of disciplines and aims to publish exceptional papers in this area.
The journal invites submissions that explicitly revolve around water desalting and its applications to various sources such as seawater, groundwater, and wastewater. It particularly encourages research on diverse desalination methods including thermal, membrane, sorption, and hybrid processes.
By providing a platform for innovative studies, Desalination aims to advance the understanding and development of desalination technologies, promoting sustainable solutions for water scarcity challenges.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


