Enhanced photorespiratory and TCA pathways by elevated CO2 to manage ammonium nutrition in tomato leaves
IF 6.1
2区 生物学
Q1 PLANT SCIENCES
引用次数: 0
Abstract
Plants grown under exclusive ammonium (NH4+) nutrition have high carbon (C) demand to sustain proper nitrogen (N) assimilation and energy required for plant growth, generally impaired when compared to nitrate (NO3−) nutrition. Thereby, the increment of the atmospheric carbon dioxide (CO2) concentration, in the context of climate change, will potentially allow plants to better face ammonium nutrition. In this work, tomato (Solanum lycopersicum L.) plants were grown under ammonium or nitrate nutrition in conditions of ambient (aCO2, 400 ppm) or elevated CO2 (eCO2, 800 ppm) atmosphere. Elevated CO2 increased photosynthesis rate and tomato shoot growth regardless of the N source. In the case of NH4+-fed leaves the positive effect of elevated CO2 occurred despite of the high tissue NH4+ accumulation. Under eCO2 ammonium nutrition triggered, among others, the modulation of genes related to C provision pathways (including carbonic anhydrase and glyoxylate cycle), antioxidant response and cell membranes protection. The enhanced photosynthate production at eCO2 facilitated C skeleton provision through the TCA cycle and anaplerotic pathways to promote amino acid synthesis. Moreover, photorespiratory activity was stimulated by eCO2 and contributed to yield serine as additional sink for NH4+ excess. Overall, these changes denote a connection between the respiratory and the photorespiratory pathways linked to ammonium nutrition. This metabolic strategy may allow crops to grow efficiently using ammonium as fertilizer in a future climate change scenario, while mitigating N losses.
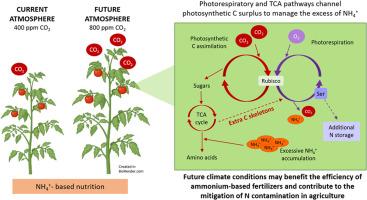
高浓度 CO2 可增强光合作用和 TCA 途径,从而管理番茄叶片中的铵营养
与硝酸盐(NO3-)营养相比,在铵营养(NH4+)条件下生长的植物需要大量的碳(C)来维持植物生长所需的氮(N)同化和能量。因此,在气候变化的背景下,大气中二氧化碳(CO2)浓度的增加有可能使植物更好地面对铵盐营养。在这项研究中,番茄(Solanum lycopersicum L.)植株在氨营养或硝酸盐营养条件下生长,生长环境为常温(aCO2,400 ppm)或二氧化碳浓度升高(eCO2,800 ppm)的大气。无论氮源如何,高浓度 CO2 都能提高光合作用速率和番茄植株的生长。在 NH4+喂养的叶片中,尽管组织中 NH4+ 积累较多,但高浓度 CO2 仍产生了积极影响。在 eCO2 条件下,铵营养引发了与 C 供给途径(包括碳酸酐酶和乙醛酸循环)、抗氧化反应和细胞膜保护相关的基因调控。在 eCO2 条件下,光合作用产生的光能促进了通过 TCA 循环和无氧代谢途径提供 C 骨架,从而促进氨基酸的合成。此外,eCO2 还刺激了光呼吸活动,并有助于产生丝氨酸作为 NH4+ 过量的额外吸收汇。总之,这些变化表明呼吸和光呼吸途径与铵营养有关。在未来气候变化的情况下,这种代谢策略可使作物利用铵作为肥料高效生长,同时减少氮的损失。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.10
自引率
3.10%
发文量
410
审稿时长
33 days
期刊介绍:
Plant Physiology and Biochemistry publishes original theoretical, experimental and technical contributions in the various fields of plant physiology (biochemistry, physiology, structure, genetics, plant-microbe interactions, etc.) at diverse levels of integration (molecular, subcellular, cellular, organ, whole plant, environmental). Opinions expressed in the journal are the sole responsibility of the authors and publication does not imply the editors'' agreement.
Manuscripts describing molecular-genetic and/or gene expression data that are not integrated with biochemical analysis and/or actual measurements of plant physiological processes are not suitable for PPB. Also "Omics" studies (transcriptomics, proteomics, metabolomics, etc.) reporting descriptive analysis without an element of functional validation assays, will not be considered. Similarly, applied agronomic or phytochemical studies that generate no new, fundamental insights in plant physiological and/or biochemical processes are not suitable for publication in PPB.
Plant Physiology and Biochemistry publishes several types of articles: Reviews, Papers and Short Papers. Articles for Reviews are either invited by the editor or proposed by the authors for the editor''s prior agreement. Reviews should not exceed 40 typewritten pages and Short Papers no more than approximately 8 typewritten pages. The fundamental character of Plant Physiology and Biochemistry remains that of a journal for original results.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


