Machine learning driven bioequivalence risk assessment at an early stage of generic drug development
IF 4.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2024-10-26
DOI:10.1016/j.ejpb.2024.114553
引用次数: 0
Abstract
Background
Bioequivalence risk assessment as an extension of quality risk management lacks examples of quantitative approaches to risk assessment at an early stage of generic drug development. The aim of our study was to develop a model-based approach for bioequivalence risk assessment that uses pharmacokinetic and physicochemical characteristics of drugs as predictors and would standardize the first step of risk assessment.
Methods
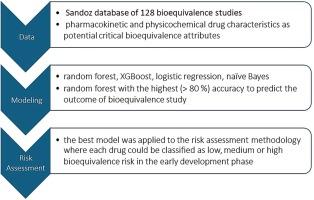
The Sandoz in-house bioequivalence database of 128 bioequivalence studies with poorly soluble drugs (23.5% non-bioequivalent) was used to train and validate the model. Four different modeling approaches, random forest, XGBoost, logistic regression and naïve Bayes, were compared.
Results
Among the best performing machine learning models, random forest was selected and optimized for the number of features, resulting in an accuracy of 84% on the test data set. The most important features for prediction were those related to solubility (dose number, acid dissociation constant), absorption and elimination rate, effective permeability, variability of pharmacokinetic endpoints, and absolute bioavailability. All features had a conceivable influence on the model predictions.
Conclusion
The model was used to develop a bioequivalence risk assessment approach to categorize drugs in early development into high, medium or low risk classes.
仿制药开发早期阶段的机器学习驱动生物等效性风险评估
背景生物等效性风险评估作为质量风险管理的延伸,在仿制药开发的早期阶段缺乏定量风险评估方法的实例。我们的研究旨在开发一种基于模型的生物等效性风险评估方法,该方法将药物的药代动力学和物理化学特征作为预测因素,并将风险评估的第一步标准化。方法使用山德士公司内部的生物等效性数据库来训练和验证模型,该数据库包含 128 项生物等效性研究,其中有溶解性较差的药物(23.5% 为非生物等效性)。结果在性能最好的机器学习模型中,随机森林被选中并对特征数量进行了优化,结果在测试数据集上的准确率达到 84%。预测中最重要的特征与溶解度(剂量数、酸解离常数)、吸收和消除率、有效渗透性、药动学终点的可变性和绝对生物利用度有关。结论该模型用于开发生物等效性风险评估方法,将早期开发药物分为高、中或低风险等级。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


