Role of catalyst porosity and acidity in nitrogen transformation during catalytic fast pyrolysis of microalgae: Study on extracted protein and model amino acids
IF 9.9
1区 工程技术
Q1 ENERGY & FUELS
引用次数: 0
Abstract
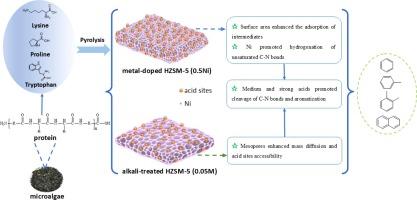
Valorizing defatted microalgae after lipid extraction maximizes the value derived from microalgae. Catalytic fast pyrolysis (CFP) of defatted microalgae effectively promotes denitrogenation, thereby advancing the sustainable production of aromatic hydrocarbons (AHs). This study explored how the intricate structures of various amino acids (lysine, proline, and tryptophan) and extracted microalgae protein influenced nitrogen transformation pathways by means of pyrolysis − gas chromatography/mass spectrometry (Py-GC/MS) at 500 °C. The roles of acidic sites and pore sizes of metal-doped (0.5Ni) and alkali-treated (0.05 M) HZSM-5 (Hydrogen Zeolite Socony Mobil-5) catalysts in denitrogenation and aromatization were focused upon. The doping of Ni led to a 2.5 % increase in medium acidity, whereas the alkaline pretreatment resulted in a 40.0 % increase in mesopore volume. The relative yields of AHs from extracted protein increased by 10.0, 10.3, and 10.5 times with the addition of HZSM-5, 0.05 M and 0.5Ni, respectively. The denitrogenation indices of the extracted protein were 0.22, 0.28 and 0.31 when HZSM-5, 0.05 M and 0.5Ni catalysts were applied, respectively. The results revealed that surface area enhanced the adsorption of intermediates from lysine, facilitating their entry into pore channels for subsequent reactions on acid sites. The formation of mesopores in the 0.05 M catalyst improved mass diffusion and accessibility of acids sites for the pyrolysis of proline and tryptophan which had a larger molecular size than lysine. A hydrogenation catalyst like Ni was crucial especially for the cleavage of N-heterocyclic amino acids with lower degree of saturation within N-containing bonds. This research provides a basic understanding of the roles that chemical structures of amino acids and catalysts synthesis play in the efficient denitrogenation and AHs production from microalgae pyrolysis.
微藻催化快速热解过程中催化剂孔隙率和酸度在氮转化中的作用:对提取的蛋白质和模型氨基酸的研究
在提取脂质后对脱脂微藻进行估值,可最大限度地提高微藻的价值。对脱脂微藻进行催化快速热解(CFP)可有效促进脱氮,从而推动芳香烃(AHs)的可持续生产。本研究通过在 500 °C 下进行热解-气相色谱/质谱分析(Py-GC/MS),探讨了各种氨基酸(赖氨酸、脯氨酸和色氨酸)和提取的微藻蛋白质的复杂结构如何影响氮转化途径。重点研究了掺杂金属(0.5 镍)和碱处理(0.05 M)的 HZSM-5(氢沸石 Socony Mobil-5)催化剂的酸性位点和孔径在脱氮和芳香化过程中的作用。掺杂镍使介质酸度增加了 2.5%,而碱性预处理则使介孔体积增加了 40.0%。添加 HZSM-5、0.05 M 和 0.5Ni 后,从提取的蛋白质中提取的 AHs 的相对产量分别增加了 10.0、10.3 和 10.5 倍。使用 HZSM-5、0.05 M 和 0.5Ni 催化剂时,提取蛋白质的脱氮指数分别为 0.22、0.28 和 0.31。结果表明,表面积增强了对赖氨酸中间产物的吸附,有利于它们进入孔道,在酸性位点上进行后续反应。0.05 M 催化剂中介孔的形成改善了质量扩散和酸性位点的可及性,有利于分子尺寸比赖氨酸大的脯氨酸和色氨酸的热解。像镍这样的氢化催化剂对于含 N 键饱和度较低的 N-杂环氨基酸的裂解尤为重要。这项研究使人们对氨基酸的化学结构和催化剂合成在微藻热解高效脱氮和生产 AHs 中的作用有了基本的了解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Energy Conversion and Management
工程技术-力学
CiteScore
19.00
自引率
11.50%
发文量
1304
审稿时长
17 days
期刊介绍:
The journal Energy Conversion and Management provides a forum for publishing original contributions and comprehensive technical review articles of interdisciplinary and original research on all important energy topics.
The topics considered include energy generation, utilization, conversion, storage, transmission, conservation, management and sustainability. These topics typically involve various types of energy such as mechanical, thermal, nuclear, chemical, electromagnetic, magnetic and electric. These energy types cover all known energy resources, including renewable resources (e.g., solar, bio, hydro, wind, geothermal and ocean energy), fossil fuels and nuclear resources.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


