Potential of a CO2-Responsive supramolecular drug-carrier system as a safer and more effective treatment for cancer
IF 8.7
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
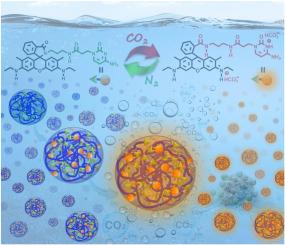
We combined carbon dioxide (CO2)-responsive cytosine-containing rhodamine 6G (Cy-R6G) as a hydrophobic anticancer agent with hydrogen-bonded cytosine-functionalized polyethylene glycol (Cy-PEG) as a hydrophilic supramolecular carrier to construct a CO2-responsive drug delivery system, with the aim of enhancing the responsiveness of the system to the tumor microenvironment and thus the overall effectiveness of anticancer therapy. Due to self-complementary hydrogen bonding interactions between cytosine units, Cy-R6G and Cy-PEG co-assemble in water to form spherical-like nanogels, with Cy-R6G effectively encapsulated within the nanogels. The nanogels exhibit several distinctive physical features, such as widely tunable nanogel size and drug loading capacity for Cy-R6G, intriguing fluorescence properties, high co-assembled structural stability in normal aqueous environments, enhanced anti-hemolytic characteristics, sensitive dual CO2/pH-responsive behavior, and precise and easily controllable CO2-induced release of Cy-R6G. Cytotoxicity assays clearly indicated that, due to the presence of cytosine receptors on the surface of cancer cells, Cy-R6G-loaded nanogels exert selective cytotoxicity against cancer cells in pristine culture medium, but do not affect the viability of normal cells. Surprisingly, in CO2-rich culture medium, Cy-R6G-loaded nanogels exhibit a further significant enhancement in cytotoxicity against cancer cells, and remain non-cytotoxic to normal cells. More importantly, a series of in vitro experiments demonstrated that compared to pristine culture medium, CO2-rich culture medium promotes more rapid selective internalization of Cy-R6G-loaded nanogels into cancer cells through cytosine-mediated macropinocytosis and thus accelerates the induction of apoptosis. Therefore, this newly developed system provides novel avenues for the development of highly effective CO2-responsive drug delivery systems with potent anticancer capabilities.
二氧化碳响应超分子药物载体系统作为更安全、更有效的癌症治疗方法的潜力
我们将二氧化碳(CO2)响应型含胞嘧啶罗丹明6G(Cy-R6G)作为疏水性抗癌剂,与氢键胞嘧啶功能化聚乙二醇(Cy-PEG)作为亲水性超分子载体相结合,构建了二氧化碳响应型给药系统,旨在增强该系统对肿瘤微环境的响应性,从而提高抗癌治疗的整体效果。由于胞嘧啶单元之间存在自互补氢键作用,Cy-R6G 和 Cy-PEG 在水中共同组装成球状纳米凝胶,Cy-R6G 被有效地包裹在纳米凝胶中。这种纳米凝胶具有多种独特的物理特性,如纳米凝胶的尺寸和 Cy-R6G 的载药量可广泛调节、荧光特性引人入胜、共组装结构在正常水环境中具有高度稳定性、抗溶血特性增强、具有灵敏的 CO2/pH 双响应行为、CO2 诱导的 Cy-R6G 释放精确且易于控制。细胞毒性试验清楚地表明,由于癌细胞表面存在胞嘧啶受体,Cy-R6G负载纳米凝胶在原始培养基中对癌细胞具有选择性细胞毒性,但不影响正常细胞的活力。令人惊讶的是,在富含二氧化碳的培养基中,Cy-R6G负载纳米凝胶对癌细胞的细胞毒性进一步显著增强,而对正常细胞仍无毒性。更重要的是,一系列体外实验表明,与纯培养基相比,富含二氧化碳的培养基能通过胞嘧啶介导的大分子胞吞作用,促进负载 Cy-R6G 的纳米凝胶更快地选择性内化到癌细胞中,从而加速诱导细胞凋亡。因此,这种新开发的系统为开发具有强效抗癌能力的高效二氧化碳响应型给药系统提供了新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


