Simultaneous targeting of TGF-β1/PD-L1 via a hydrogel-nanoparticle system to remodel the ECM and immune microenvironment for limiting adhesion formation
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Adhesion seriously affects the recovery of tendon gliding function. Our group previously found that inhibition of TGF-β1, which is closely related to adhesion formation, effectively attenuated adhesions but did not eliminate them, suggesting that there may be other mechanisms involved in adhesion formation. In this study, we considered that uncontrolled and excessively proliferating fibroblasts undergo immune escape, which aggravates the deposition of extracellular matrix during the adhesion formation. We found that the expression of the immune checkpoint PD-L1 was significantly elevated after injury and may be involved in adhesion formation. Therefore, we intended to silence both TGF-β1 and PD-L1 to improve the immune advantage in the microenvironment after flexor tendon injury to further reduce adhesion. We constructed the nanoparticle/TGF-β1 or/and PD-L1 siRNAs complexes and verified their high biocompatibility and high transfection efficiency. We found that CD8+ T cells had a greater killing effect on the excessively proliferating cells that were transfected with nanoparticle/TGF-β1 or/and PD-L1 siRNAs. The hydrogel-nanoparticle/TGF-β1 or/and PD-L1 siRNAs system could effectively improve the gliding function of the tendons without weakening the mechanical properties in injured rat FDL tendon and chicken FDP tendon models. In addition, the potential of CD8+ T cells to encircle the adhesion cells on the tendon surface was observed, which resulted in increased levels of cell apoptosis. Thus, our study confirmed that combined knockdown of TGF-β1 and PD-L1 could activate immunodominance after flexor tendon repair and provided a potential treatment to limit adhesion formation and improve gliding function.
Statement of Significance
Adhesion seriously affects the recovery of tendon gliding function. TGF-β1 is related to adhesion formation as it regulates the production of extracellular matrix. We found that excessively proliferated fibroblasts might undergo immune escape, which aggravated the deposition of extracellular matrix. Therefore, we constructed a hydrogel-nanoparticle/TGF-β1 and PD-L1 siRNAs system for silencing TGF-β1 and PD-L1 to improve the immune advantage in the microenvironment after tendon injury. This system could improve the gliding function of tendons without weakening the mechanical property and increase the killing effect of CD8+ T cells. Combined knockdown of TGF-β1 and PD-L1 could activate immunodominance after tendon repair and provide a potential treatment to limit adhesion formation.
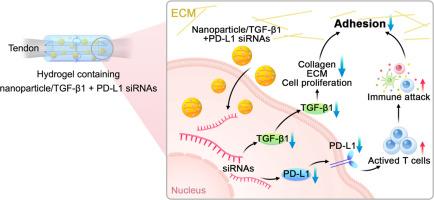
通过水凝胶-纳米粒子系统同时靶向 TGF-β1/PD-L1,重塑 ECM 和免疫微环境以限制粘附的形成。
粘连严重影响肌腱滑行功能的恢复。我们小组之前发现,抑制与粘连形成密切相关的 TGF-β1,能有效减轻粘连,但并不能消除粘连,这表明粘连的形成可能还有其他机制。在本研究中,我们认为不受控制和过度增殖的成纤维细胞会发生免疫逃逸,从而在粘连形成过程中加剧细胞外基质的沉积。我们发现,免疫检查点 PD-L1 的表达在损伤后显著升高,可能参与了粘连的形成。因此,我们打算同时抑制 TGF-β1 和 PD-L1,以改善屈肌腱损伤后微环境中的免疫优势,进一步减少粘连。我们构建了纳米颗粒/TGF-β1 或/和 PD-L1 siRNA 复合物,并验证了其高生物相容性和高转染效率。我们发现,CD8+ T 细胞对转染了纳米颗粒/TGF-β1 或/和 PD-L1 siRNA 的过度增殖细胞有更大的杀伤作用。水凝胶-纳米颗粒/TGF-β1 或/和 PD-L1 siRNA 系统能有效改善损伤大鼠 FDL 肌腱和鸡 FDP 肌腱模型中肌腱的滑动功能,而不会削弱其机械性能。此外,还观察到 CD8+ T 细胞有可能包围肌腱表面的粘附细胞,从而导致细胞凋亡水平升高。因此,我们的研究证实,联合敲除 TGF-β1 和 PD-L1 可激活屈肌腱修复后的免疫优势,并为限制粘连形成和改善滑行功能提供了一种潜在的治疗方法。意义声明:粘连严重影响肌腱滑行功能的恢复。TGF-β1 与粘连的形成有关,因为它能调节细胞外基质的生成。我们发现,过度增殖的成纤维细胞可能会发生免疫逃逸,从而加剧细胞外基质的沉积。因此,我们构建了一种水凝胶-纳米颗粒/TGF-β1 和 PD-L1 siRNAs 系统,用于沉默 TGF-β1 和 PD-L1,以改善肌腱损伤后微环境中的免疫优势。该系统能在不削弱肌腱机械性能的情况下改善肌腱的滑动功能,并提高 CD8+ T 细胞的杀伤效果。联合敲除 TGF-β1 和 PD-L1 可以激活肌腱修复后的免疫优势,并为限制粘连形成提供一种潜在的治疗方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


