Cells and signals of the leukemic microenvironment that support progression of T-cell acute lymphoblastic leukemia (T-ALL)
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Current intensified chemotherapy regimens have significantly increased survival rates for pediatric patients with T-cell acute lymphoblastic leukemia (T-ALL), but these treatments can result in serious adverse effects; furthermore, patients who are resistant to chemotherapy or who relapse have inferior outcomes, together highlighting the need for improved therapeutic strategies. Despite recent advances in stratifying T-ALL into molecular subtypes with distinct driver mutations, efforts to target the tumor-intrinsic genomic alterations critical for T-ALL progression have yet to translate into more effective and less toxic therapies. Ample evidence now indicates that extrinsic factors in the leukemic microenvironment are critical for T-ALL growth, infiltration, and therapeutic resistance. Considering the diversity of organs infiltrated by T-ALL cells and the unique cellular components of the microenvironment encountered at each site, it is likely that there are both shared features of tumor-supportive niches across multiple organs and site-specific features that are key to leukemia cell survival. Therefore, elucidating the distinct microenvironmental cues supporting T-ALL in different anatomic locations could reveal novel therapeutic targets to improve therapies. This review summarizes the current understanding of the intricate interplay between leukemia cells and the diverse cells they encounter within their tumor microenvironments (TMEs), as well as opportunities to therapeutically target the leukemic microenvironment. T-cell Acute lymphoblastic leukemia (T-ALL) is an aggressive, predominantly pediatric, cancer arising from excessive proliferation of transformed immature T cells in the thymus. Despite treatment advancements, patients experience significant side effects of chemotherapy, and those who fail to respond or relapse face poor outcomes, highlighting the need for more effective treatment strategies. This review examines how the tumor microenvironment supports growth and survival of T-ALL cells. The authors discuss current evidence indicating that interactions between T-ALL cells and the TME contribute to disease progression and treatment resistance in various organs, including the bone marrow, central nervous system, and spleen. These studies suggest that targeting interactions between T-ALL cells and the TME could offer new treatment opportunities. The authors conclude that disrupting the supportive signals of the TME could enhance existing therapies and reduce disease relapse. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
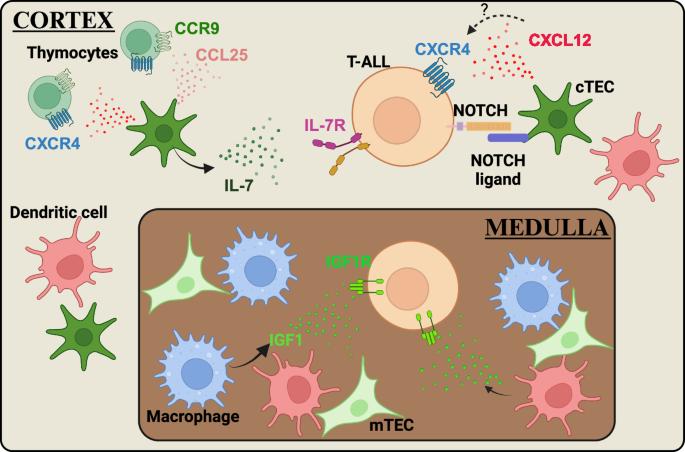
支持 T 细胞急性淋巴细胞白血病 (T-ALL) 进展的白血病微环境细胞和信号。
目前的强化化疗方案大大提高了T细胞急性淋巴细胞白血病(T-ALL)儿科患者的存活率,但这些治疗可能会导致严重的不良反应;此外,对化疗耐药或复发的患者预后较差,这些都凸显了改进治疗策略的必要性。尽管最近在将 T-ALL 分为具有不同驱动突变的分子亚型方面取得了进展,但针对对 T-ALL 进展至关重要的肿瘤内在基因组改变所做的努力尚未转化为更有效、毒性更低的疗法。现在有大量证据表明,白血病微环境中的外在因素对 T-ALL 的生长、浸润和耐药性至关重要。考虑到 T-ALL 细胞浸润器官的多样性以及每个部位微环境中独特的细胞成分,多个器官的肿瘤支持龛既可能存在共享特征,也可能存在对白血病细胞存活至关重要的特定部位特征。因此,阐明不同解剖部位支持T-ALL的不同微环境线索可揭示新的治疗靶点,从而改善疗法。本综述总结了目前对白血病细胞与它们在肿瘤微环境(TME)中遇到的各种细胞之间错综复杂的相互作用的理解,以及针对白血病微环境的治疗机会。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


