Autoinjector optimization through cavitation response and severity minimization
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
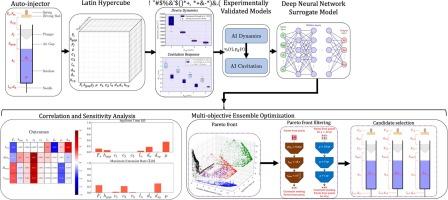
Abrupt acceleration of the syringe of an autoinjector (AI) upon rod-plunger impact may induce undesired severe cavitation events and impose extraneous stresses upon the device, leading to device failure. Cavitation results from a rapid and significant pressure drop in a liquid, leading to the formation and growth of small vapor-filled cavities. Upon collapse, these cavities generate an intense shock wave that may lead to protein aggregation and device container damage and shatter. Since the maximum acceleration of the syringe depends upon the operating conditions of the AI, the severity of cavitation will likewise depend on the operating conditions of the AI. Likewise, injection time and ensuring proper needle displacement before drug release also depend on operating conditions, making optimization of the autoinjector a multiobjective optimization problem.
Therefore, in this study, optimization of an autoinjector to limit cavitation severity is pursued via an experimentally validated computational model for cavitation in spring-driven autoinjectors. Our goal is to locate AI design configurations that balance maximizing device performance and patient comfort and minimizing the risks of device damage and severe cavitation upon actuation.
Relevant parameters of interest are the drive spring force, air gap height, solution viscosity, friction between the rod and spring, frictional force on the plunger, rates of change of frictional force on the plunger, elasticity of plunger, viscosity of the plunger, and initial displacement between the plunger and the driving rod. The kinematics of the syringe barrel, needle displacement (travel distance) at the start of drug delivery, and injection time are gathered using an experimentally validated autoinjector kinematics model. At the same time, cavitation bubble dynamics are resolved using an experimentally validated cavitation model that takes the temporal displacement of the syringe and temporal air gap pressure as inputs.
We use our experimentally validated models to explore the parameter space and understand the driving factors of our desired outcomes. Subsequently, we pose the design problem as a multi-objective optimization problem and develop a deep neural network surrogate model supplemented with iterative learning to speed up optimization. A variance-based sensitivity analysis was performed to determine the sensitivity and influence of design parameters on the outcomes, and the main contributors to the outcomes of interest were isolated. Using a multi-objective optimization framework, we located 300 + successful candidates and evaluated them through uncertainty analysis to identify three promising candidates that meet all criteria for drug viscosities of interest. Finally, we show that this methodology can be used to conduct hypothesis testing, leading to novel design configurations.
通过气蚀响应和严重程度最小化优化自动注射器。
自动注射器(AI)的注射器在受到杆柱塞撞击时突然加速,可能会诱发不希望发生的严重空化现象,并对设备造成额外的应力,从而导致设备故障。气蚀是由于液体中的压力急剧下降,导致充满蒸汽的小空腔形成和增大。塌陷时,这些空腔会产生强烈的冲击波,可能导致蛋白质聚集、装置容器损坏和破碎。由于注射器的最大加速度取决于人工智能的操作条件,因此空化的严重程度也同样取决于人工智能的操作条件。同样,注射时间和确保药物释放前针头的适当位移也取决于操作条件,因此自动注射器的优化是一个多目标优化问题。因此,在本研究中,我们将通过经实验验证的弹簧驱动自动注射器气蚀计算模型,对自动注射器进行优化,以限制气蚀的严重程度。我们的目标是找到人工智能设计配置,在设备性能和患者舒适度最大化与设备损坏和启动时严重气蚀风险最小化之间取得平衡。相关参数包括驱动弹簧力、气隙高度、溶液粘度、杆和弹簧之间的摩擦力、柱塞上的摩擦力、柱塞上摩擦力的变化率、柱塞的弹性、柱塞的粘度以及柱塞和驱动杆之间的初始位移。通过实验验证的自动注射器运动学模型,收集了注射器枪管的运动学特性、开始给药时针头的位移(移动距离)和注射时间。与此同时,我们还利用实验验证的空化模型解析了空化气泡动力学,该模型将注射器的时间位移和时间气隙压力作为输入。我们利用经过实验验证的模型来探索参数空间,了解我们所期望的结果的驱动因素。随后,我们将设计问题作为一个多目标优化问题,并开发了一个深度神经网络代用模型,辅以迭代学习来加快优化速度。我们进行了基于方差的敏感性分析,以确定设计参数对结果的敏感性和影响,并分离出导致相关结果的主要因素。利用多目标优化框架,我们找到了 300 + 个成功的候选方案,并通过不确定性分析对它们进行了评估,最终确定了三个有希望的候选方案,它们符合相关药物粘度的所有标准。最后,我们展示了这种方法可用于进行假设检验,从而产生新的设计配置。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


