Computational and experimental analysis of Luteolin-β-cyclodextrin supramolecular complexes: Insights into conformational dynamics and phase solubility
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2024-10-29
DOI:10.1016/j.ejpb.2024.114569
引用次数: 0
Abstract
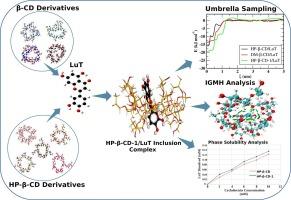
Investigating the structural stability of poorly-soluble luteolin (LuT) after encapsulation within cyclodextrins (CDs) is crucial for unlocking the therapeutic potential of LuT bioactive molecule. Herein, native and modified β-CD were employed to investigate LuT inclusion complex formation. Molecular mechanics (MM) and quantum mechanics (QM) were utilized for structural dynamics analysis. Microsecond timescale MD simulations yielded insights into LuT-CD interactions. The binding affinity between LuT and selected β-CDs was assessed by calculating the binding free energy using MM-PBSA and umbrella sampling simulations. The MM-PBSA results indicated that Heptakis-O-(2-hydroxypropyl)-β-CD (HP-β-CD) (−82.59+/-11.67 kJ/mol) and Di-O-methyl-β-CD (DM-β-CD) (−54.01+/-11.07 kJ/mol) exhibited good binding affinity for LuT. Subsequently, derivative screening of HP-β-CD revealed that only 2-HP-β-CD (HP-β-CD-1)/LuT (−21.38 kJ/mol) displayed a superior binding free energy (obtained from umbrella sampling) than HP-β-CD/LuT (−16.55 kJ/mol) inclusion complex. We conducted QM calculations on the top three inclusion complexes namelly HP-β-CD, DM-β-CD, and HP-β-CD-1 employing wB97X-D/6–311 + G(d,p) model chemistry to strengthen the MM results. The computational analysis aligns with experimental findings (phase solubility analysis), validating HP-β-CD-1 as most effective cavitand molecule for improving the solubility of LuT. This study offers critical structural insights for developing novel HP-β-CD derivatives with enhanced host capacity to encapsulate guest molecules efficiently.
木犀草素-β-环糊精超分子复合物的计算和实验分析:对构象动力学和相溶解性的见解。
研究溶解性差的木犀草素(LuT)被环糊精(CD)包裹后的结构稳定性,对于挖掘 LuT 生物活性分子的治疗潜力至关重要。本文采用原生和改性β-CD来研究LuT包合物的形成。分子力学(MM)和量子力学(QM)被用于结构动力学分析。微秒级的 MD 模拟揭示了 LuT 与 CD 之间的相互作用。通过使用 MM-PBSA 和伞状采样模拟计算结合自由能,评估了 LuT 与所选 β-CD 之间的结合亲和力。MM-PBSA 结果表明,Heptakis-O-(2-羟基丙基)-β-CD(HP-β-CD)(-82.59+/-11.67 kJ/mol)和 Di-O-methyl-β-CD (DM-β-CD)(-54.01+/-11.07 kJ/mol)与 LuT 具有良好的结合亲和力。随后,对 HP-β-CD 进行衍生筛选后发现,只有 2-HP-β-CD (HP-β-CD-1)/LuT (-21.38 kJ/mol) 的结合自由能(通过伞状取样获得)高于 HP-β-CD/LuT (-19.15 kJ/mol) 包合物。我们采用 wB97X-D/6-311 + G(d,p) 化学模型对排名前三的复合物命名为 HP-β-CD、DM-β-CD 和 HP-β-CD-1 进行了 QM 计算,以加强 MM 结果。计算分析结果与实验结果(相溶解度分析)一致,验证了 HP-β-CD-1 是提高 LuT 溶解度最有效的空穴剂分子。这项研究为开发新型 HP-β-CD 衍生物提供了重要的结构见解,这些衍生物具有更强的宿主能力,能有效地包裹客体分子。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


