UCl3-Type Solid Electrolytes: Fast Ionic Conduction and Enhanced Electrode Compatibility
IF 14.7
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
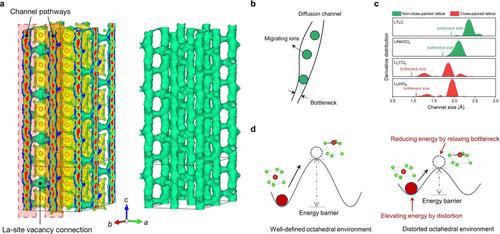
Figure 1. Origin of the superionic conduction of UCl3-type SEs with the non-close-packed framework. (a) Li+ probability density, represented by green isosurfaces from AIMD simulations in the vacancy-contained LaCl3 lattice. Reproduced with permission from reference (21). Copyright 2023 the author(s), under exclusive license to Springer Nature Limited. (b) Schematic of diffusion channel. Reproduced with permission from reference (24). Copyright 2024 John Wiley and Sons. (c) Diffusion channel size distribution of Li3YCl6, Li3InCl6, LiNbOCl4, and UCl3-type Li0.388Ta0.238La0.475Cl3 (LTLC). Reproduced with permission from reference (24). Copyright 2024 John Wiley and Sons. (d) Schematic illustration of the effects of inherent distortion on energy landscape. Reproduced with permission from reference (24). Copyright 2024 John Wiley and Sons. Figure 2. Ionic conductivity values at room temperature of crystalline chloride SEs, including conventional close-packed LixMyCln SEs and UCl3-type LaCl3-based SEs. (1−4,10−14,21) Reproduced with permission from reference (21). Copyright 2023 the author(s), under exclusive license to Springer Nature Limited. Figure 3. UCl3-type SEs with a more stable interface toward lithium metal anode. (a) Depth-dependent La 3d5/2 X-ray photoelectron spectroscopy (XPS) spectra of the interface of Li0.388Ta0.238La0.475Cl3 SE after 50 h of cycling. Reproduced with permission from reference (21). Copyright 2023 by Springer Nature Limited. (b) Depth-dependent La 3d5/2 XPS spectra of the interface of Li0.388Ta0.238La0.475Cl3 SE after 50 h of cycling. Reproduced with permission from reference (21). Copyright 2023 the author(s), under exclusive license to Springer Nature Limited. (c) Voltage profile of a Li/Li0.388Ta0.238La0.475Cl3/Li symmetric cell cycled under a current density of 0.2 mA cm–2 and areal capacity of 1 mAh cm–2 at 30 °C. Insets: corresponding magnified voltage profiles indicate steady Li plating/stripping voltages. Reproduced with permission from reference (21). Copyright 2023 the author(s), under exclusive license to Springer Nature Limited. (d) La 3d5/2 (left) and Zr 3d (right) XPS spectra of the Li|Li0.8Zr0.25La0.5Cl2.7O0.3 interface after 500 h cycling, respectively. Reproduced with permission from reference (23). Copyright 2024 Royal Society of Chemistry. (e) Comparison of the critical current density (CCD) of Li metal symmetric cells with different solid electrolytes (Ga-LLZO (Li6.4Ga0.2La3Zr2O12); LAGP (Li1.5Al0.5Ge1.5(PO4)3); Ta-LLZO (Li6.5La3Zr1.5Ta0.5O12); PEO:Mg(ClO) (PEO:Mg(ClO4)2); LiBFSIE-LLZO (LiBFSIE-Li7La3Zr2O12); PEO:LLZTO; O–LiPSBr(O-doped Li6PS4.7O0.3Br); LiPS-0.5LiI (Li3PS4-0.5LiI); rod-like LiPSCl (Li6PS5Cl)). Reproduced with permission from reference (23). Copyright 2024 Royal Society of Chemistry. Density. The central elements (lanthanide metals La, Ce, Sm, etc.) and commonly used doping elements (Ta, Zr, etc.) for UCl3-type SEs are heavy, often leading to a high density of UCl3-type SEs over 2.5 g cm–3, much higher than that of sulfides (usually less than 2 g cm–3). To ensure a low weight ratio of nonactive materials in the cathode and SE layer in the whole solid battery for higher energy density, (31) doping elements with low atom numbers (e.g., Ca, Mg, and Al et al.) are preferred. Optimization in the anode stabilization mechanism. Though LaCl3-based SEs have shown better interface compatibility with the lithium metal anode than conventional LixMyCln, the stabilization mechanisms are still not thoroughly identified. Meanwhile, the capacity of around 1 mAh cm–2 is insufficient to meet the demand of practical applications (usually over 3 mAh cm–2). A deeper understanding of interface evolution, accompanied by an artificial interfacial layer to enhance anode interface stability, is needed. Atmosphere tolerance. Similar to conventional LixMyCln, due to easy reaction or combination with water, (32) the atmosphere tolerance of UCl3-type SEs needs enhancement to restrain the performance loss during synthesis, store, film-forming process and ASSLB fabrication. Y.C.Y, J.D.L, and H.B.Y discussed the topic and proposed the outline. Y.C.Y organized and wrote the draft. H.B.Y revised the manuscript. Yi-Chen Yin is now a postdoctoral researcher at the University of Science and Technology of China. He obtained his Bachelor’s degree from the China University of Mining and Technology in 2017 and his Ph.D. degree from the University of Science and Technology of China in 2022. His interests are in new halide solid electrolytes with high ionic conductivity and good electrode interface stability. Jin-Da Luo is now an M.S. candidate at the University of Science and Technology of China. He obtained his Bachelor’s degree from Xiangtan University in 2021. His research focuses on the computational modeling and simulation of ion transport within the lattice of solid electrolytes. Hong-Bin Yao received his BS degree from the University of Science and Technology of China in 2006. Then, he pursued his Ph.D. degree at the Hefei National Laboratory for Physical Sciences at the microscale under the supervision of Professor Shu-Hong Yu. After receiving his Ph.D. degree in 2011, he joined Professor Yi Cui’s group at Stanford University as a postdoc. In 2015, he finished his postdoc work and joined the University of Science and Technology of China as a professor. His group focuses on functional metal halide crystalline materials and related device applications. We acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 22475235, 22325505, 52073271, 22305236), the USTC Research Funds of the Double First-Class Initiative (YD2060002034), the Collaborative Innovation Program of Hefei Science Center, CAS (Grant No. 2022HSC-CIP018), and the China Postdoctoral Science Foundation (Grant No. 2023M733375 and 2023T160619). This article references 32 other publications. This article has not yet been cited by other publications.
UCl3 型固体电解质:快速离子传导和增强电极兼容性
图 1.具有非紧密堆积框架的 UCl3 型 SE 超离子传导的起源。(a) 在含有空位的 LaCl3 晶格中,用 AIMD 模拟得到的绿色等值面表示的 Li+ 概率密度。经授权转载自参考文献 (21)。作者版权所有 2023 年,Springer Nature Limited 独家授权。(b) 扩散通道示意图。经授权转载自参考文献 (24)。John Wiley and Sons 公司版权所有 2024。(c) Li3YCl6、Li3InCl6、LiNbOCl4 和 UCl3 型 Li0.388Ta0.238La0.475Cl3 (LTLC) 的扩散通道尺寸分布。经授权转载自参考文献 (24)。John Wiley and Sons 公司版权所有,2024 年。(d) 内在畸变对能量分布的影响示意图。经授权转载自参考文献 (24)。约翰-威利父子公司版权所有 2024 年。图 2.晶体氯化物 SE(包括传统的紧密堆积 LixMyCln SE 和基于 UCl3 型 LaCl3 的 SE)在室温下的离子电导率值。(1-4,10-14,21) 经授权转载自参考文献 (21)。作者版权所有 2023 年,Springer Nature Limited 独家授权。图 3.对锂金属阳极具有更稳定界面的 UCl3 型 SE。(a) 循环 50 小时后,Li0.388Ta0.238La0.475Cl3 SE 接口的深度依赖性 La 3d5/2 X 射线光电子能谱 (XPS) 光谱。经授权转载自参考文献 (21)。施普林格自然有限公司版权所有 2023 年。(b) 循环 50 小时后,Li0.388Ta0.238La0.475Cl3 SE 接口的深度依赖性 La 3d5/2 XPS 光谱。经授权转载自参考文献 (21)。作者版权所有 2023 年,Springer Nature Limited 独家授权。(c) 锂/锂 0.388Ta0.238La0.475Cl3/Li 对称电池在 0.2 mA cm-2 电流密度和 1 mAh cm-2 单位容量条件下于 30 °C 循环的电压曲线。插图:相应的放大电压曲线表示稳定的锂电镀/剥离电压。经授权转载自参考文献 (21)。作者版权所有 2023 年,Springer Nature Limited 独家授权。(d) 在循环 500 小时后,Li|Li0.8Zr0.25La0.5Cl2.7O0.3 接口的 La 3d5/2(左)和 Zr 3d(右)XPS 光谱。经参考文献 (23) 授权转载。版权归英国皇家化学学会所有,2024 年。(e) 采用不同固体电解质的锂金属对称电池临界电流密度 (CCD) 的比较:Ga-LLZO(Li6.4Ga0.2La3Zr2O12);LAGP(Li1.5Al0.5Ge1.5(PO4)3);Ta-LLZO(Li6.5La3Zr1.5Ta0.5O12);PEO:Mg(ClO)(PEO:Mg(ClO4)2);LiBFSIE-LLZO(LiBFSIE-Li7La3Zr2O12);PEO:LLZTO;O-LiPSBr(O-掺杂Li6PS4.7O0.3Br);LiPS-0.5LiI(Li3PS4-0.5LiI);棒状LiPSCl(Li6PS5Cl))。经授权转载自参考文献 (23)。版权 2024 年英国皇家化学学会。密度。UCl3 型 SE 的中心元素(镧系金属 La、Ce、Sm 等)和常用掺杂元素(Ta、Zr 等)都很重,通常导致 UCl3 型 SE 的密度超过 2.5 g cm-3,远高于硫化物的密度(通常低于 2 g cm-3)。为了确保整个固态电池中阴极和 SE 层的非活性材料重量比低,以获得更高的能量密度,(31) 低原子数的掺杂元素(如 Ca、Mg 和 Al 等)是首选。优化阳极稳定机制。虽然与传统的 LixMyCln 相比,基于 LaCl3 的 SE 显示出与锂金属负极更好的界面兼容性,但其稳定机制仍未完全确定。同时,1 mAh cm-2 左右的容量不足以满足实际应用的需求(通常超过 3 mAh cm-2)。我们需要更深入地了解界面演化,并辅以人工界面层来增强阳极界面的稳定性。大气耐受性。与传统的 LixMyCln 相似,由于容易与水反应或结合(32),UCl3 型 SE 的大气耐受性需要提高,以抑制合成、储存、成膜过程和 ASSLB 制造过程中的性能损失。Y.C.Y、J.D.L 和 H.B.Y讨论了该课题并提出了大纲。Y.C.Y 组织并撰写草稿。H.B.Y 修改了手稿。尹以琛现为中国科学技术大学博士后研究员。2017 年获中国矿业大学学士学位,2022 年获中国科学技术大学博士学位。他的研究方向是具有高离子电导率和良好电极界面稳定性的新型卤化物固体电解质。罗金达现为中国科学技术大学硕士研究生。他于 2021 年获得湘潭大学学士学位。他的研究重点是固体电解质晶格内离子传输的计算建模与模拟。姚宏斌于 2006 年获得中国科学技术大学学士学位。 之后,他在合肥物理科学国家实验室攻读微尺度博士学位,师从俞书宏教授。2011 年获得博士学位后,他进入斯坦福大学崔毅教授课题组做博士后。2015 年,他结束博士后工作,加入中国科学技术大学任教授。他的研究小组主要研究功能性金属卤化物晶体材料及相关器件应用。感谢国家自然科学基金(批准号:22475235、22325505、52073271、22305236)、中国科学技术大学双一流建设研究基金(YD2060002034)、中科院合肥科学中心协同创新计划(批准号:2022HSC-CIP018)和中国博士后科学基金(批准号:2023M733375和2023T160619)的资助。本文引用了其他 32 篇文章。本文尚未被其他出版物引用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


