Anti-seizure effects of norepinephrine-induced free fatty acid release
IF 27.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
The brain’s ability to rapidly transition between sleep, quiet wakefulness, and states of high vigilance is remarkable. Cerebral norepinephrine (NE) plays a key role in promoting wakefulness, but how does the brain avoid neuronal hyperexcitability upon arousal? Here, we show that NE exposure results in the generation of free fatty acids (FFAs) within the plasma membrane from both astrocytes and neurons. In turn, FFAs dampen excitability by differentially modulating the activity of astrocytic and neuronal Na+, K+, ATPase. Direct application of FFA to the occipital cortex in awake, behaving mice dampened visual-evoked potential (VEP). Conversely, blocking FFA production via local application of a lipase inhibitor heightened VEP and triggered seizure-like activity. These results suggest that FFA release is a crucial step in NE signaling that safeguards against hyperexcitability. Targeting lipid-signaling pathways may offer a novel therapeutic approach for seizure prevention.
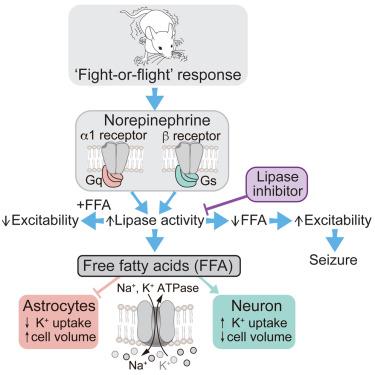
去甲肾上腺素诱导游离脂肪酸释放的抗癫痫作用
大脑在睡眠、安静的清醒状态和高度警觉状态之间快速转换的能力是非凡的。大脑去甲肾上腺素(NE)在促进觉醒中起着关键作用,但大脑如何避免神经元在觉醒时过度兴奋呢?在这里,我们发现暴露于 NE 会导致星形胶质细胞和神经元的质膜内产生游离脂肪酸(FFA)。反过来,游离脂肪酸通过不同程度地调节星形胶质细胞和神经元的 Na+、K+、ATP 酶的活性来抑制兴奋性。在清醒的行为小鼠枕叶皮层直接施用反式脂肪酸可抑制视觉诱发电位(VEP)。相反,通过在局部应用脂肪酶抑制剂来阻断反式脂肪酸的产生,则会增强视觉诱发电位并引发癫痫样活动。这些结果表明,FFA 释放是 NE 信号传导的关键步骤,可防止过度兴奋。以脂质信号通路为靶点可能为预防癫痫发作提供一种新的治疗方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


