Selective Hydride Interstitials Induced in a High-Entropy Lanthanide Oxyhydride
IF 4.4
2区 化学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
High-entropy materials have gained significant interest in many applications, but structural investigations of the effect on anions in the crystal structure are still scarce. Here, we study the effect of multicomponent cation disorder in the case of mixed-anion compounds. The distribution of mixed anions among various coordination sites is important given their implications for properties such as ionic conductivity and bulk diffusion in catalysis. Structural analysis in the fluorite-type (La,Ce,Pr,Nd,Y)H1.5O0.75 reveals that the disordered cationic effects create new interstitial sites, occupied selectively by hydride despite oxide and hydride disorder in other compositions and sites. In contrast, single-lanthanide oxyhydrides of analogous anion content, such as LaH1.5O0.75, or SmH2O0.5 lack the complex interstitial structure. Hydride ion conductivity measurements and bond valence sum energy maps show a considerably low activation energy of hydride migration due to the additional interstitial sites induced by high entropy. Such interstitials can be crucial in applications that involve hydride ion diffusion, such as ammonia synthesis catalysis and solid-state ionics, as further high-entropy compositions are explored.
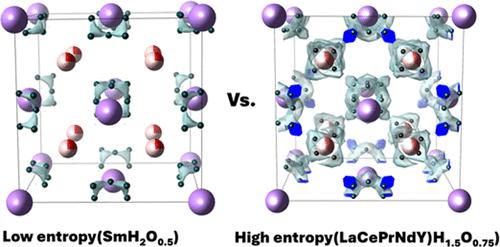
高熵镧系酸酐中诱导的选择性氢化物间隙
高熵材料在许多应用领域都获得了极大的关注,但有关晶体结构中阴离子影响的结构研究仍然很少。在此,我们研究了多组分阳离子无序对混合阴离子化合物的影响。混合阴离子在各个配位点之间的分布非常重要,因为它们对离子导电性和催化过程中的体扩散等性质都有影响。对萤石型 (La,Ce,Pr,Nd,Y)H1.5O0.75的结构分析表明,无序阳离子效应产生了新的间隙位点,尽管在其他成分和位点中存在氧化物和氢化物无序现象,但氢化物却选择性地占据了这些位点。相比之下,阴离子含量类似的单镧系氢氧化合物,如 LaH1.5O0.75 或 SmH2O0.5 则缺乏复杂的间隙结构。氢化物离子电导率测量结果和键价和能图显示,由于高熵引起的额外间隙位点,氢化物迁移的活化能相当低。随着对更多高熵成分的探索,这些间隙在涉及氢化物离子扩散的应用中(如氨合成催化和固态离子技术)可能至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Polymer Materials
Multiple-
CiteScore
7.20
自引率
6.00%
发文量
810
期刊介绍:
ACS Applied Polymer Materials is an interdisciplinary journal publishing original research covering all aspects of engineering, chemistry, physics, and biology relevant to applications of polymers.
The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrates fundamental knowledge in the areas of materials, engineering, physics, bioscience, polymer science and chemistry into important polymer applications. The journal is specifically interested in work that addresses relationships among structure, processing, morphology, chemistry, properties, and function as well as work that provide insights into mechanisms critical to the performance of the polymer for applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


