Unveiling electrochemical insights of lithium manganese oxide cathodes from manganese ore for enhanced lithium-ion battery performance
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
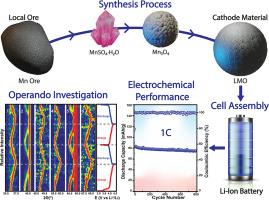
Implementing manganese-based electrode materials in lithium-ion batteries (LIBs) faces several challenges due to the low grade of manganese ore, which necessitates multiple purification and transformation steps before acquiring battery-grade electrode materials, increasing costs. At present, most Lithium Manganese Oxide (LMO) materials are synthesized using electrolytic manganese dioxide, and the development of new processes, such as hydrometallurgical processes is important for achieving a cost-effective synthesis of LMO materials. In this work, we develop a full synthesis process of LMO materials from manganese ore, through acid leaching, forming manganese sulfate monohydrate (MnSO4·H2O), an optimized thermal decomposition (at 900, 950 or 1000 °C) producing different Mn3O4 materials and a solid-state reaction, achieving the synthesis of LMO. The latter was used as a cathode material for LIB exhibiting a specific capacity comparable to the state-of-the-art LMO cathode with a remarkable cycling stability of 800 cycles with <20 % in capacity loss. These performances were attributed to the excellent redox reversibility of the LMO cathode, characterized by voltammetry and in operando and in situ characterization by Raman and XRD.
揭示从锰矿中提取氧化锰锂阴极的电化学原理,提高锂离子电池性能
在锂离子电池(LIB)中使用锰基电极材料面临着一些挑战,因为锰矿石品位低,在获得电池级电极材料之前必须经过多个提纯和转化步骤,从而增加了成本。目前,大多数锂锰氧化物(LMO)材料都是利用电解二氧化锰合成的,而开发新工艺(如湿法冶金工艺)对于实现 LMO 材料的低成本合成非常重要。在这项工作中,我们从锰矿石中开发了一套完整的 LMO 材料合成工艺,通过酸浸出形成一水硫酸锰(MnSO4-H2O),优化热分解(在 900、950 或 1000°C 下)产生不同的 Mn3O4 材料,以及固态反应,实现 LMO 的合成。后者被用作锂离子电池的阴极材料,显示出与最先进的 LMO 阴极相当的比容量和显著的循环稳定性,可循环 800 次,容量损失小于 20%。这些性能归功于 LMO 阴极出色的氧化还原性,并通过伏安法以及拉曼和 XRD 进行了操作和原位表征。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


