Integration across biophysical scales identifies molecular and cellular correlates of person-to-person variability in human brain connectivity
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Brain connectivity arises from interactions across biophysical scales, ranging from molecular to cellular to anatomical to network level. To date, there has been little progress toward integrated analysis across these scales. To bridge this gap, from a unique cohort of 98 individuals, we collected antemortem neuroimaging and genetic data, as well as postmortem dendritic spine morphometric, proteomic and gene expression data from the superior frontal and inferior temporal gyri. Through the integration of the molecular and dendritic spine morphology data, we identified hundreds of proteins that explain interindividual differences in functional connectivity and structural covariation. These proteins are enriched for synaptic structures and functions, energy metabolism and RNA processing. By integrating data at the genetic, molecular, subcellular and tissue levels, we link specific biochemical changes at synapses to connectivity between brain regions. These results demonstrate the feasibility of integrating data from vastly different biophysical scales to provide a more comprehensive understanding of brain connectivity. Integration of postmortem molecular and dendritic spine morphological measurements enables the detection of microscale molecules associated with person-to-person variability in macroscale brain connectivity estimated from antemortem neuroimaging.

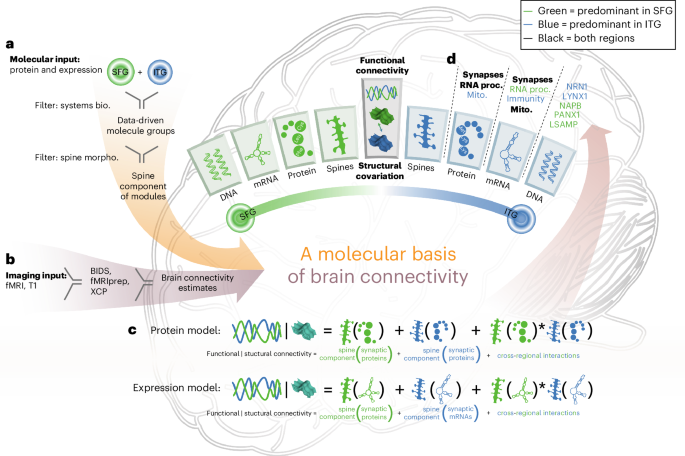
跨生物物理尺度的整合确定了人脑连通性中人与人之间差异的分子和细胞相关性
从分子到细胞,从解剖学到网络水平,大脑连通性产生于不同生物物理尺度的相互作用。迄今为止,跨这些尺度的综合分析进展甚微。为了弥补这一差距,我们从一个独特的 98 人队列中收集了死前神经影像学和遗传学数据,以及死后额上回和颞下回的树突棘形态计量学、蛋白质组学和基因表达数据。通过整合分子和树突棘形态学数据,我们发现了数百种能解释个体间功能连接和结构共变差异的蛋白质。这些蛋白质富集于突触结构和功能、能量代谢和 RNA 处理。通过整合遗传、分子、亚细胞和组织水平的数据,我们将突触的特定生化变化与脑区之间的连接性联系起来。这些结果证明了整合来自不同生物物理尺度的数据以更全面地了解大脑连通性的可行性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


