Approach to extended polycyclic aromatic hydrocarbons by benzannulation and intramolecular cyclization; improvement of the reaction conditions by microwave irradiation
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The extended π-conjugated compounds 1–8 were synthesized by the benzannulation of the corresponding alkyne derivatives followed by Pd-catalyzed intramolecular cyclization. For the Pd-catalyzed intramolecular cyclization, microwave irradiation reduced the amount of the Pd catalyst and the reaction time. Moreover, this protocol can be achieved to prepare the extended and twisted π-conjugated molecule 19, having anthracene, helicene, and picene skeletons.
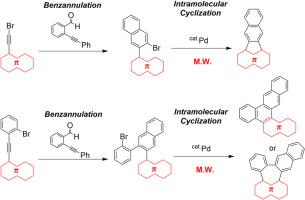
通过苯annulation 和分子内环化反应制备扩展的多环芳烃;利用微波辐照改善反应条件
扩展的π-共轭化合物 1-8 是通过相应炔衍生物的苯并环化和钯催化的分子内环化合成的。在钯催化的分子内环化反应中,微波辐照减少了钯催化剂的用量和反应时间。此外,这种方法还可以制备出具有蒽、螺旋烯和皮烯骨架的扩展和扭曲的π-共轭分子 19。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


