Targeted antimicrobial self-assembly peptide hydrogel with in situ bio-mimic remineralization for caries management
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
The single-function agents with wide-spectrum activity which tend to disturb the ecological balance of oral cavity cannot satisfy dental treatment need. A multi-functional agent with specifically targeted killing property and in situ remineralization is warranted for caries management. A novel multi-functional agent (8DSS-C8-P-113) consisting of three domains, i.e., a non-specific antimicrobial peptide (AMP) (P-113), a competence stimulating peptide (C8), and an enhancing remineralization domain (8DSS), is fabricated and evaluated in this study. The findings demonstrates that 2 μM mL−1 of 8DSS-C8-P-113 eliminates planktonic Streptococcus mutans (S. mutans) without disrupting the oral normal flora. At a concentration of 8 μM mL−1, it exhibits the ability to prevent S. mutans' adhesion. Furthermore, 8DSS-C8-P-113 self-assembles a hydrogel state at the higher concentration of 16 μM mL−1. This hydrogel self-adheres on the tooth surface, resisting acid attack, eradicating S. mutans’ biofilm, and inducing mineralization in order to facilitate the repair of demineralized dental hard tissue. Its significant effectiveness in reducing the severity of dental caries is also demonstrated in vivo in a rat model. This study suggests that the multi-functional bioactive AMP 8DSS-C8-P-113 is a promising agent to specifically target pathogen, prevent tooth demineralization, and effectively induce in situ bio-mimic remineralization for the management of dental caries.
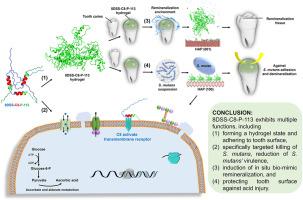
具有原位生物模拟再矿化功能的靶向抗菌自组装肽水凝胶,用于龋齿管理
单一功能的制剂具有广谱活性,容易破坏口腔的生态平衡,无法满足牙科治疗的需要。在龋病治疗中,需要一种具有特定靶向杀灭特性和原位再矿化功能的多功能制剂。本研究制作并评估了一种新型多功能制剂(8DSS-C8-P-113),它由三个结构域组成,即非特异性抗菌肽(AMP)(P-113)、能力刺激肽(C8)和增强再矿化结构域(8DSS)。研究结果表明,2 μM mL-1 的 8DSS-C8-P-113 能消除浮游的变异链球菌(S. mutans),而不会破坏口腔正常菌群。当浓度为 8 μM mL-1 时,它还能阻止变异链球菌的粘附。此外,8DSS-C8-P-113 还能在 16 μM mL-1 的较高浓度下自组装成水凝胶状态。这种水凝胶能自我附着在牙齿表面,抵御酸性物质的侵蚀,清除变异棒状杆菌的生物膜,并诱导矿化,从而促进脱矿牙齿硬组织的修复。它在降低龋齿严重程度方面的显著效果也在大鼠模型中得到了验证。这项研究表明,多功能生物活性 AMP 8DSS-C8-P-113 是一种很有前景的制剂,可专门针对病原体,防止牙齿脱矿,并有效诱导原位生物模拟再矿化,从而治疗龋齿。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


